CAMBRIDGE, Mass., Nov. 4, 2008 – A new scaffold approach developed by a team of researchers from the Massachusetts Institute of Technology (MIT) could one day be used to mend a broken heart.
The idea is that living heart cells or stem cells seeded onto an accordion-like honeycomb scaffold would develop into a patch of cardiac tissue that could be used to treat congenital heart defects, or aid the recovery of tissue damaged by a heart attack. The biodegradable scaffold would be gradually absorbed into the body, leaving behind new tissue.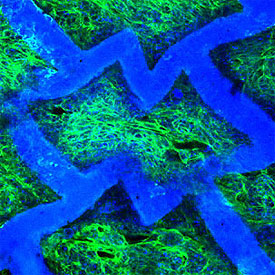
Confocal micrograph of an accordion-like honeycomb scaffold with cultured rat heart cells (scaffold is colored blue; seeded, living heart cells are colored green with blue nuclei). Original magnification = 250X. Image courtesy of G.C. Engelmayr, Jr.
With the hope of mimicking nature’s lessons more closely, the researchers created a scaffold with directionally dependent structural and mechanical properties using a laser similar to that used for eye surgery.
This is the first scaffold to be explicitly designed to match the structural and mechanical properties of native heart tissue. As a result, it has several advantages over previous cardiac tissue engineering scaffolds. According to the MIT team, their approach has applications to other types of engineered tissues.
"In the long term we'd like to have a whole library of scaffolds for different tissues in need of repair," said Lisa E. Freed, a principal research scientist in the Harvard-MIT Division of Health Sciences and Technology (HST). “Each scaffold could be tailor-made with specific structural and mechanical properties. We're already on the way to a few other examples." 
Principal research scientist with Harvard-MIT Health Sciences Lisa Freed and George Engelmayr Jr., post-doc in Harvard-MIT Health Sciences report new scaffold (shown on monitor) for tissue engineering of the heart. Photo courtesy of Donna Coveney.
"Previous scaffolds did not necessarily possess structural or mechanical properties consistent with the native myocardial (heart muscle) structure," said George C. Engelmayr Jr., HST postdoctoral fellow, adding that heart muscle is directionally dependent, meaning its cells are aligned in specific directions.
The scaffold has three principal advantages over its predecessors. First, its mechanical properties closely match those of native heart tissue. For example, it is stiffer when stretched circumferentially as compared to longitudinally.
Engelmayr found that he could essentially "dial in" specific mechanical properties for the polymer scaffold by varying the time it is allowed to set, or cure. He noted that with this ability, coupled with the flexibility of the laser technique, "we might be able to come up with even better pore shapes with better mechanical properties."
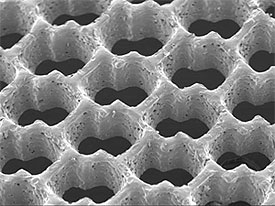
Scanning electron micrograph of an accordion-like honeycomb scaffold for cardiac tissue engineering. Original magnification =100 X. Image courtesy of G.C. Engelmayr, Jr.
In a second advantage, the team found that a patch of tissue created from neonatal rat heart cells cultured on the scaffold showed directionally dependent electrophysiological properties similar to native tissue. In other words, when an electrical field was applied the engineered patch contracted more readily in one direction than in another.
In a third advantage, "the scaffold itself has an intrinsic ability to guide the orientation of cultured heart cells," Freed said (in 2004, Freed was part of another MIT team that showed that heart cells cultured on a traditional scaffold could also be coaxed into alignment, but only with electrical stimulation).
The researchers note that the scaffold used in the experiments described above has some limitations, such as the fact that is too thin to address reconstruction of full-thickness myocardium. However they have already begun addressing those problems by creating new honeycomb scaffolds that, among other things, allow much thicker, multi-layered tissue structures.
Other researchers and colleagues are Robert Langer, MIT Institute professor; Mingyu Cheng, currently at Children's Hospital Boston; Christopher J. Bettinger, currently at Stanford University; and Jeffrey T. Borenstein of the Charles Stark Draper Laboratory.
This work was sponsored by the National Institutes of Health, NASA, and Draper Laboratory.
For more information, visit: http://www.mit.edu/