A laser device that injects relatively large-sized "cargo" into mammalian cells at high speed could aid the study of disease.
Called a biophotonic laser-assisted surgery tool, or BLAST, the device was developed by engineers and doctors at the University of California, Los Angeles (UCLA).
It is capable of delivering nanoparticles, enzymes, antibodies, bacteria and other objects up to 1 μm in size into as many as 100,000 cells per minute. That's significantly faster than current techniques using micropipettes, which can reach about one cell per minute.
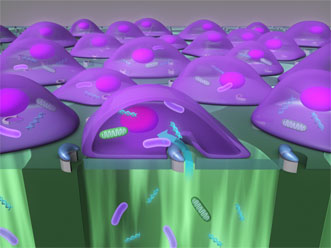
BLAST, the biophotonic laser-assisted surgery tool, delivers objects smaller than 1 μm in size into as many as 100,000 cells per minute. Courtesy of Pei-Yu "Eric" Chiou.
Other approaches for injecting materials into cells – such as using viruses as delivery vehicles or chemical methods – are only useful for small molecules, which are typically several nanometers in length.
BLAST is a silicon chip with an array of micron-wide holes, each surrounded by an asymmetric, semicircular coating of titanium. Underneath the holes is a well of liquid that includes the particles to be delivered.
Laser pulses heat the titanium coating, which instantly boils the water layer outside the cell, creating bubbles that explode and form pores in the cell membranes. The reaction takes about 1 μs.
The particle-filled liquid is then driven gently through the pores by pressurized flow before the cell membranes reseal.
The key to the technique's success is the instantaneous and precise incision of the cell membrane, said Pei-Yu "Eric" Chiou, associate professor of mechanical and aerospace engineering and of bioengineering at UCLA's Henry Samueli School of Engineering and Applied Science.

"The faster you cut, the fewer perturbations you have on the cell membrane," he said.
Inserting large cargo into cells could lead to scientific research that was previously not possible. For example, the ability to deliver mitochondria could alter cells' metabolism and help researchers study diseases caused by mutant mitochondrial DNA.
It also could help scientists dissect the function of genes involved in the lifecycle of pathogens that invade cells and understand cell-defense mechanisms.
"Now it doesn’t matter the size or type of material you want to deliver," Chiou said. "You can just push all of it into the cell."
"The new information learned from these types of studies could assist in identifying pathogen targets for drug development, or provide fundamental insight on how the pathogen-host interaction enables a productive infection or effective cellular response to occur," said Dr. Michael Teitell, chief of UCLA's division of pediatric and developmental pathology.
Because BLAST can rapidly deliver cargo to 100,000 cells, a single chip could provide enough data for a statistical analysis of how cells respond in an experiment, the researchers said.
Funding came from the University of California Discovery Biotechnology Award, the National Institutes of Health, NanoCav and the National Science Foundation.
The research was published in Nature Methods (doi: 10.1038/nmeth.3357).
For more information, visit www.ucla.edu.