WILLIAM BURGESS, POWER TECHNOLOGY INC.
The only constants in life may be death and taxes, but many would argue that regulatory change is another one of life’s certainties. Proof of this can be seen in the FDA-initiated transition from the current laser manufacturing safety guidance, Laser Notice No. 50, to a new framework, Laser Notice No. 56, which is set to take effect at the end of this year.
To maintain safety standards, the FDA has issued a series of laser notices as guidance for manufacturers to meet compliance requirements. As defined by the FDA, a laser notice is a document that outlines specific requirements and safety guidelines for laser products. Alongside the Federal Laser Product Performance Standards, outlined in 21 Code of Regulations (CFR) 1010 and 1040, these notices provide regulatory guidance to laser device manufacturers, ensuring consumer safety and product quality. As such, they are crucial to the design, labeling, and manufacturing of laser products intended for sale in the U.S. Certain notices are intended to harmonize with international regulations, enabling U.S. manufacturers to compete more effectively in the global marketplace.
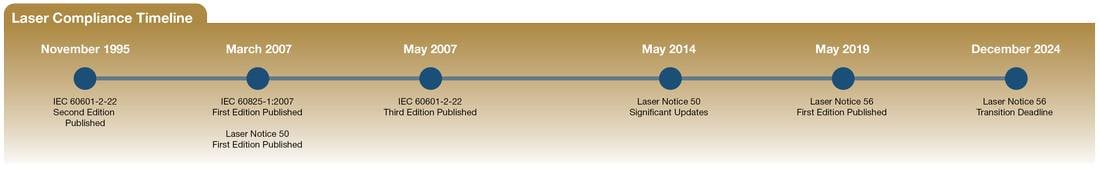
A timeline showing the evolution of laser regulations. Courtesy of Power Technology Inc.
Introduced in May 2019, Laser Notice No. 56, as compared with Laser Notice No. 50, places a stronger emphasis on harmonization and compliance with the latest International Electrotechnical Commission (IEC) standards. Most notably, the new framework prioritizes IEC 60825-1 Ed. 3 (Safety of laser products — Part 1: Equipment classification and requirements); and IEC 60601-2-22 Ed. 3.1 (Particular requirements for basic safety and essential performance of surgical, cosmetic, therapeutic, and diagnostic laser equipment). The FDA plans to withdraw Laser Notice No. 50 on Dec. 31, 2024, and Laser Notice No. 56 will provide regulatory guidance in this area instead.
This upcoming shift affects various stakeholders in the photonics industry. Laser manufacturers that stay ahead of the regulatory curve by proactively implementing these updates can offer significant advantages to their customers. For this reason, it is beneficial to consider how laser providers can effectively anticipate and adapt to regulatory changes.
Considerations for laser manufacturers
The responsibility for implementing these regulatory changes rests mainly on laser manufacturers, which must ensure that their laser products meet the new guidelines before the deadline. Laser products manufactured until Dec. 31, 2024, may continue to use the laser safety labels described in Laser Notice No. 50. Existing products with these older labels will remain acceptable for use. As manufacturers transition to the guidance given in Laser Notice No. 56, lasers manufactured after Jan. 1, 2025, must adopt the new labeling requirements.
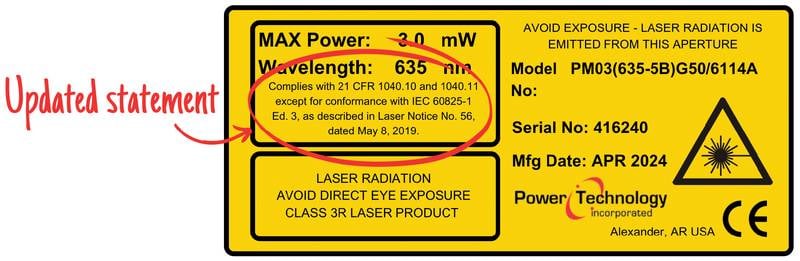

Following the implementation of Laser Notice No. 56, laser labeling requirements will undergo changes. Updated label verbiage (top). Previous label format (bottom). Courtesy of Power Technology Inc.
Depending on the type of laser, compliance with the newer laser notice may be as simple as updating the laser’s certification label. Additionally, compliance with Laser Notice No. 56 must be documented and communicated to the FDA’s Center for Devices and Radiological Health through a supplemental report submission.
For laser users
Because there is no requirement to update labels on older lasers, laser users have no need to worry about this shift. Simply put, these lasers are just as safe as they have always been. But, with the purchase of any new lasers, completing the necessary research on the laser manufacturer is important. If a user cannot determine the manufacturer’s name and where the laser was manufactured, it is advisable to seek an alternative supplier. Users should search for a company that has a long-established track record and appreciates laser safety as well as regulatory compliance. When purchasing a new laser, always review the safety label and ensure that the new language, provided below, is present.
Adjustments for laser safety officers
Laser safety officers may want to review the upcoming labeling requirements to ensure that all new lasers received after Jan. 1, 2025, are compliant with Laser Notice No. 56. There is no need to re-evaluate lasers manufactured before Jan. 1, 2025. The existing product and hazard labels are still acceptable. But new lasers should have compliant labels with one of the following statements:
Complies with FDA performance standards for laser products except for conformance with IEC 60825-1 Ed. 3, as described in Laser Notice No. 56, dated May 8, 2019.
Or:
Complies with 21 CFR 1040.10 and 1040.11 except for conformance with IEC 60825-1 Ed. 3, as described in Laser Notice No. 56, dated May 8, 2019.
Changes for purchasing companies
Manufacturers that use lasers as a component of a larger system may want to implement incoming inspections to ensure that their laser provider is aware of the latest regulations (i.e., the labeling change, etc.). If a manufacturer is not using lasers from a domestic manufacturer, they may need to work with their supplier to ensure that products entering the U.S. are compliant with U.S. federal laws. The photonics industry has seen enough supply chain disruption during the last few years. Avoiding a U.S. customs package “HOLD” while importing noncompliant products is easy to avoid through the proper diligence or the use of a reputable domestic laser supplier.
As the laser industry evolves, so must its safety standards. The withdrawal of Laser Notice No. 50 and the adoption of Laser Notice No. 56 represent a step forward by emphasizing global regulatory alignment while maintaining the safety of laser users in the U.S. and abroad.
For laser manufacturers, it is time to embrace the transition and ensure that their laser products meet the highest safety benchmarks. For the last 55 years, companies, such as Power Technology Inc., have positioned themselves at the forefront of laser technology and regulatory compliance. All laser users are encouraged to keep their lasers shining bright, but always within safe limits.