Enabled by innovative imaging mechanisms, fast scanning technologies, and high throughput, the microscopy technique can be used to image tissues that are sensitive to dynamic changes.
JUNJIE YAO AND VAN TU NGUYEN, DUKE UNIVERSITY
When Alexander Graham Bell discovered the photoacoustic effect in the 1880s, he might not have envisioned that this technology would ultimately be successfully implemented in biomedical imaging applications such as neuroscience, dermatology, and cancer biology. In the photoacoustic effect, the excitation light is absorbed by molecules in a sample, and the absorbed photon energy slightly elevates the
local temperature via nonradiative relaxation, which eventually induces a pressure wave propagating as ultrasound (Figure 1a).
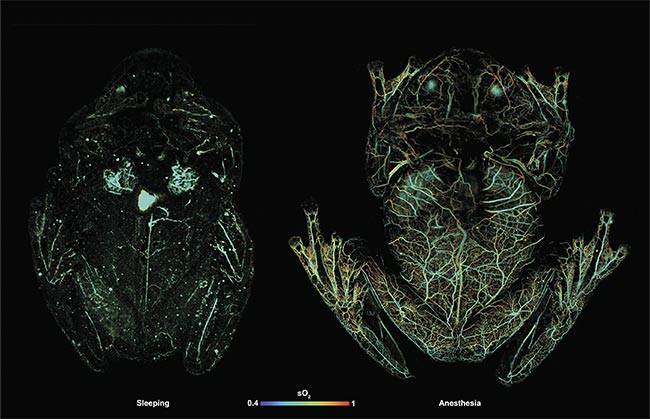
Photoacoustic microscopy (PAM) images of glass frog hemodynamics during anesthesia (right) and resting (left). Courtesy of Junjie Yao/Duke University.
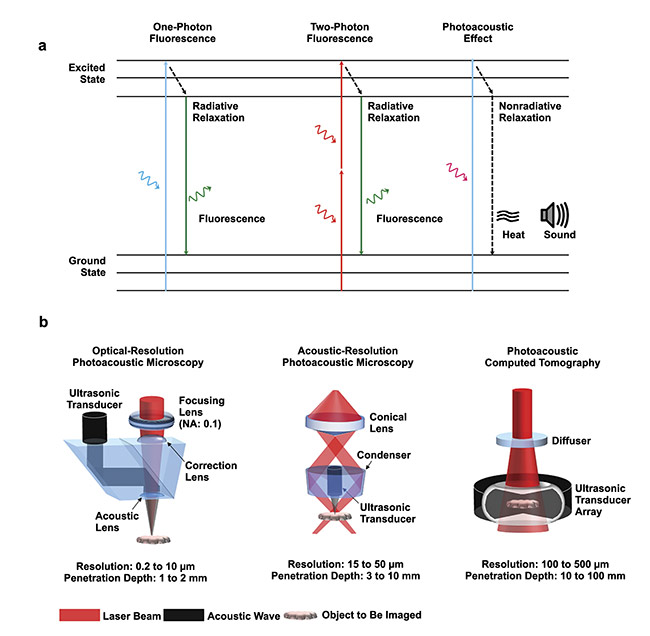
Figure 1. The principle of the photoacoustic effect compared with one-photon and two-photon fluorescence emission (a). The major implementations of
photoacoustic tomography (PAT), with resolution and penetration depth (b). Courtesy of Junjie Yao/Duke University.
This process is similar to the fluorescence emission process, in which the absorbed photon energy is converted to fluorescent photons via radiative relaxation. Unlike the widespread availability of electrical power with which to develop the telephone in the 1880s, Bell had only the sunlight-powered photoacoustic effect to utilize for communication. This made the endeavor very challenging, mostly due to the lack of light stability. But as laser technologies have rapidly advanced as reliable and powerful light sources, they have enabled the use of photoacoustics in materials science, telecommunications, chemical engineering, and biomedicine. Among the many applications of the photoacoustic effect, photoacoustic tomography (PAT), which is also referred to as optoacoustic tomography, has made the most significant impact on both fundamental science and clinical health care. PAT has emerged as one of the fastest-growing imaging technologies and has the capacity to enable early cancer diagnosis, functional brain imaging, drug delivery monitoring, and the guidance of interventional procedures1,2. For example, in 2021,
Seno Medical Instruments Inc. introduced the first FDA-approved PAT system to the market for breast cancer diagnosis, paving the way for more commercial PAT systems to receive regulatory clearance.
PAT components
A typical PAT system includes a short-pulsed laser for efficient photoacoustic wave generation; a wideband ultrasonic transducer for photoacoustic signal detection; a data acquisition system for signal amplification, filtering, and digitization; and a computational system for data processing, image reconstruction, and functional quantification. So far, PAT has been implemented using two major image formation methods (Figure 1b). The first method, direct image formation (commonly referred to as photoacoustic microscopy, or PAM), is based on mechanical scanning of a focused excitation light beam and a focused single-element ultrasound transducer. PAM can be further classified into optical-resolution PAM (OR-PAM) and acoustic-resolution PAM (AR-PAM), depending on the focal spot size of the optical excitation and acoustic detection. The second method, inverse reconstruction image formation (commonly referred to as photoacoustic computed tomography, or PACT), is based on wide-field light illumination and parallel acoustic detection by a multielement ultrasound transducer array. Compared with PAM, PACT typically has a higher imaging speed and greater penetration but lower spatial resolutions. Thus, PAM is more often used for biomedical studies at cellular or even subcellular levels, while PACT is more suitable for imaging biological samples at tissue or organ levels.
PAM in modern use
Harnessing concurrent advancements in physics, chemistry, mathematics, and computer science, PAM has experienced its fastest development in the last decade, driven by the innovations in research labs and the growing market need in the commercial sector. So far, PAM has been widely used for numerous biomedical applications in which high spatial resolution is needed within the first several millimeters of biological tissues, such as in functional brain imaging, circulating tumor cell detection, and skin cancer diagnosis. The expansion of PAM applications has been fueled by advancements in almost every key system component, such as light sources with higher power; higher repetition rate; wider wavelength tuning range; and lower cost, as well as novel ultrasound detectors with higher sensitivity, larger frequency bandwidth, and lower cost.
Biological functions occur on a wide range of temporal and spatial scales, which requires PAM technologies to provide best-matched imaging speeds and wide field of view (FOV). For PAM to achieve a high imaging speed over a large FOV, with high detection sensitivity, it is critical to maintain the confocal alignment of the optical excitation and acoustic detection. In recent microscopy experiments, 1D or 2D water-immersible resonant microelectromechanical systems (MEMS) scanning mirrors were utilized that confocally steer both the excitation laser beam and the emitted acoustic beam with a moderate FOV of ~3 × 3 sq mm and uncompromised detection sensitivity.
However, it is challenging for the MEMS scanning mirrors to provide a larger FOV. To address the trade-off between the scanning speed and scanning range of MEMS scanners, a recent study using a water-immersible polygon mirror scanner in OR-PAM achieved a 2D imaging rate of 1.2 kHz over a 12 × 12 sq mm FOV3. Unlike the resonant MEMS mirror, the polygon scanner can maintain its large scanning range at different scanning frequencies, which is critical for imaging large organs, such as the blood oxygenation change of the whole mouse cortex in response to hypoxia (Figure 2a)4 and the circulating red blood cell storage in the livers of resting glass frogs (Figure 2b)5.
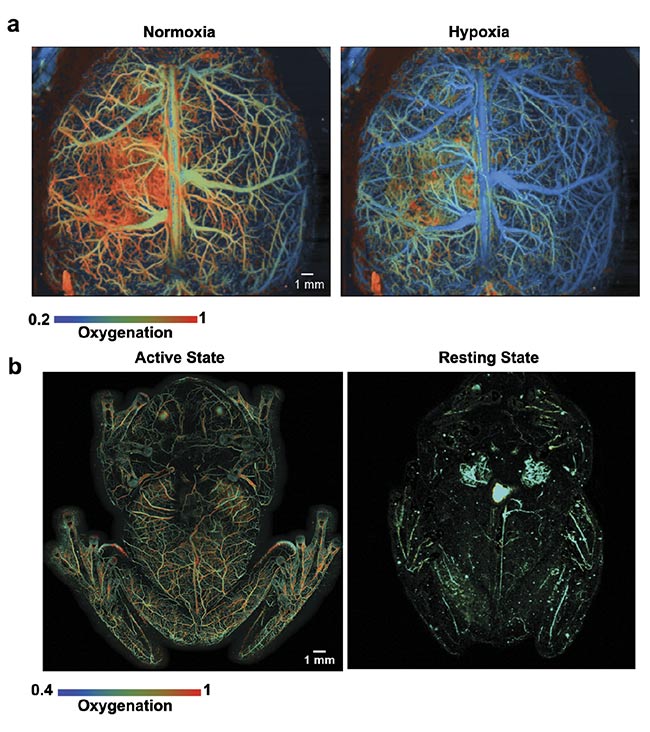
Figure 2. High-speed PAM images of mouse brain hemodynamics in response to the onset of hypoxia. The blood oxygenation level is color-coded from high (red-orange) to low (green-blue) (a). High-speed PAM images of glass frog hemodynamics during anesthesia (left) and resting (right). The color of the image indicates the oxygenation level (b). Courtesy of Junjie Yao/Duke University.
The fast scanning-based approaches have significantly improved the imaging speed of PAM systems, but speed remains limited by the laser repetition rate. This limitation is particularly true for high-speed OR-PAM, in which sparse sampling has become a necessary compromise. To relax the requirement on the laser’s pulse repetition rate, one solution is nonscanning photoacoustic imaging based on an ergodic relay. This technique can simultaneously encode all of the photoacoustic signals from a large FOV according to their unique time-delay characteristics, which can achieve a frame rate of 2 kHz over a field of view of 6 × 7.5 sq mm. This technique has been applied to image the blood pulse wave velocity and track the circulation of melanoma cells in mouse brain.
Limited by slow imaging speed and bulky system size, desktop PAM is mostly applied on small animals under anesthesia or on human subjects while the targeted region is fixed (such as the arm, hand, or finger) to minimize the motion artifacts. Enabled by the elevated imaging speed and the resultant high imaging throughput, it has become possible to implement miniaturized PAM to image otherwise challenging targets prone to motion artifacts, such as the brain functions of freely moving animals, the longitudinal monitoring of rare circulating tumor cells in melanoma patients, and skin cancer screening of difficult regions such as arms, necks, and backs.
In recent years, various PAM systems have been developed for hand-held (Figure 3a), wearable (Figure 3b)6, and even head-mounted applications (Figure 3c)7,8, thanks to the advancements in high-speed scanning methods and soft electronics fabrications. All of these technical innovations have allowed the miniaturization of PAM systems with much improved portability but without sacrificing the key imaging performance. For example, Qian Chen and colleagues reported a wearable PAM system that is small enough to be mounted on the head of a freely moving mouse8. A miniaturized MEMS scanning mirror provided high-speed, high-resolution imaging of the brain’s hemodynamic activities during and post ischemia challenge. Remarkably, the motion artifacts were negligible during the seven-day imaging period.
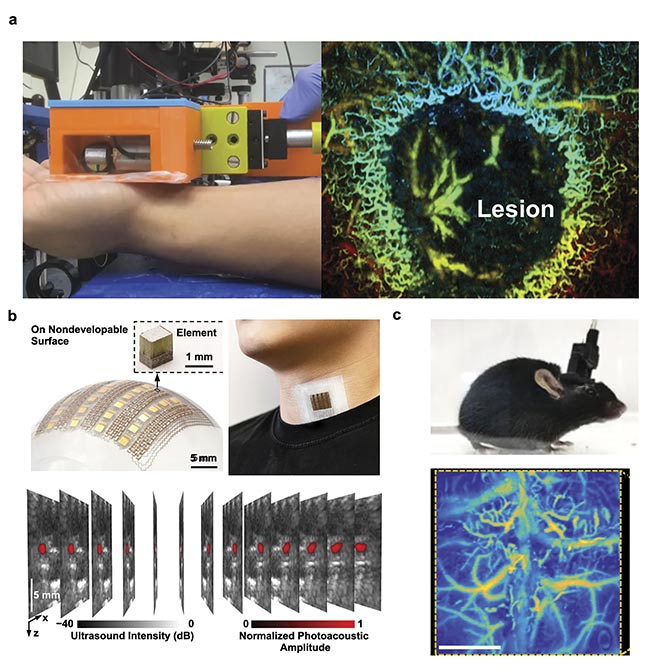
Figure 3. A hand-held PAM system (a, left) in operation for mapping a skin lesion (a, right). Courtesy of Junjie Yao/Duke University. A soft electronics-enabled wearable PAT system that can be patched to the neck’s skin surface to measure the hemodynamics of the internal jugular vein (b). Adapted with permission from Reference 6. A head-mounted PAM system fixed on a mouse head (c, top) and a representative image of cortical vasculature (c, bottom). Adapted with
permission from Reference 7.
Overall, the technical advancements in PAM have exceeded the limitations in biomedical imaging necessitated by the traditional trade-off between imaging speed, FOV, and detection sensitivity. Powered by superfast lasers and novel scanning mechanisms, PAM has achieved high-speed, high-resolution imaging over a field of view similar to that of a conventional microscope, and research has shown that PAM can be used to monitor the neurovascular coupling of an entire mouse cortex. The substantial improvement in imaging throughput has enabled implementations of PAM in portable and wearable formats, allowing for longitudinal monitoring of biological functions in freely moving animals or awake patients, with negligible motion artifacts.
In the future, PAM should grow quickly in terms of both system development and biomedical applications. With more commercially available PAM systems tailored for clinical practice, particularly in skin disease diagnosis, more and more users will benefit from the systems’ unique capabilities. Developing low-cost PAM systems is an important step that can help to improve the technique’s accessibility by a larger biomedical community, and many research groups are pursuing development along these lines. In particular, low-cost light sources such as laser diodes and light-emitting diodes, once they are widely available, will significantly reduce the system cost of PAM and accelerate the technical translation to clinical practice. The success of commercial PAM products in the marketplace should, in turn, provide strong incentives for key industrial partners to develop products that are specially optimized for PAT, such as high-power, high-speed lasers with relaxed coherence.
Enabled by these updated system components, which are often the bottlenecks in PAM technology, according to system developers, the next wave of technological breakthroughs will naturally follow. These could include real-time PAM systems for human imaging, such as skin cancer screening; high-speed, high-resolution PAM of neuronal activities enabled by novel voltage- or calcium-sensitive photoacoustic probes; highly compact endoscopic and intravascular PAM enabled by optical ultrasound sensors; and single-organelle or single-molecule photoacoustic imaging enabled by superresolution mechanisms.
Meet the authors
Junjie Yao is an assistant professor of biomedical engineering in the Photoacoustic Imaging Lab at Duke University and a faculty network member of the Duke Institute for Brain Sciences. He has a doctorate in biomedical engineering from Washington University in St. Louis; email: [email protected].
Van Tu Nguyen is a postdoctoral scholar of biomedical engineering in the Photoacoustic Imaging Lab at Duke University. He has a doctorate in biomedical/medical engineering from Pukyong National University; email: [email protected].
Acknowledgments
This work was sponsored by the United States National Institutes of Health (NIH) grants R01 EB028143, R01 NS111039, RF1 NS115581 (BRAIN Initiative); National Science Foundation (NSF) CAREER award 2144788; and Chan Zuckerberg Initiative Grant (2020-226178) (all awarded to Junjie Yao).
References
1. J. Yao and L.V. Wang (2018). Recent progress in photoacoustic molecular imaging. Curr Opin Chem Biol, Vol. 45, pp. 104-112.
2. J. Yao and L.V. Wang (2021). Perspective on fast-evolving photoacoustic tomography.
J Biomed Opt, Vol. 26, No. 6, p. 060602.
3. B. Lanet al. (2018). High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed Opt Express, Vol. 9, No. 10, pp. 4689-4701.
4. X. Zhu et al. (2022). Real-time whole-brain imaging of hemodynamics and oxygenation at micro-vessel resolution with ultrafast wide-field photoacoustic microscopy. Light Sci Appl, Vol. 11, No. 1, pp. 1-15.
5. C. Taboada et al. (2022). Glassfrogs conceal blood in their liver to maintain transparency. Science, Vol. 378, No. 6626, pp. 1315-1320.
6. X. Gao et al. (2022). A photoacoustic patch for three-dimensional imaging of hemoglobin and core temperature. Nat Commun,
Vol. 13, No. 1, p. 7757.
7. H. Guo et al. (2021). Detachable head-mounted photoacoustic microscope in freely moving mice. Opt Lett, Vol. 46, No. 24,
pp. 6055-6058.
8. Q. Chen et al. (2019). Wearable optical resolution photoacoustic microscopy. J Biophotonics, Vol. 12, No. 8.