Proteins and other molecules are often tightly packed together inside a living cell. These dense clusters can be difficult to image because the fluorescent labels used to make them visible can’t wedge themselves in between the molecules. Researchers at MIT developed a method to overcome this limitation by expanding a cell or tissue sample prior to labeling, effectively de-crowding the molecules and making them more accessible to fluorescent tags.
The method builds on a widely used technique known as expansion microscopy, which was previously developed at MIT, and enables scientists to visualize molecules and cellular structures that have never been seen before.
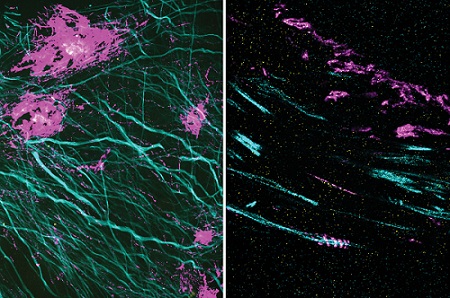
In these two images, the magenta signal is the amyloid-beta nanostructure, revealed by post-expansion staining. The left image shows the thread-like patterns of amyloid-beta nanoclusters, and the right image shows the helical structure of amyloid-beta, which was not revealed using previous techniques. Courtesy of Zhuyu Peng and Jinyoung Kang/MIT.
“It’s becoming clear that the expansion process will reveal many new biological discoveries," said Edward Boyden, professor of biological engineering and brain and cognitive sciences at MIT. "If biologists and clinicians have been studying a protein in the brain or another biological specimen, and they’re labeling it the regular way, they might be missing entire categories of phenomena.”
Using this technique, Boyden and colleagues showed that they could image a nanostructure found in the synapses of neurons. They also imaged the structure of Alzheimer’s-linked amyloid-beta plaques in greater detail than has been previously possible.
“Our technology, which we named expansion revealing, enables visualization of these nanostructures, which previously remained hidden, using hardware easily available in academic labs,” said Deblina Sarkar, an assistant professor in the MIT Media Lab and one of the lead authors of the study.
Imaging a specific protein or other molecule inside a cell requires labeling with a fluorescent tag carried by an antibody that binds to the target. Antibodies are about 10 nm long, while typical cellular proteins are usually about 2 to 5 nm in diameter, so if the target proteins are too densely packed, the antibodies can’t get to them.
This has been an obstacle to traditional imaging as well as to the original version of expansion microscopy, which Boyden first developed in 2015. In the original version, researchers attached fluorescent labels to molecules of interest before they expanded the tissue. The labeling was done first, in part because the researchers had to use an enzyme to chop up proteins in the sample so that the tissue could be expanded.

With so many more proteins accessible for labeling, the researchers were able to identify tiny cellular structures within synapses, the connections between neurons that are densely packed with proteins. They labeled and imaged seven different synaptic proteins, which allowed them to visualize, in detail, “nanocolumns” consisting of calcium channels aligned with other synaptic proteins. These nanocolumns, which are believed to help make synaptic communication more efficient, were first discovered in 2016 in the lab of Thomas Blanpied, a University of Maryland professor and a senior author of the study.
“This technology can be used to answer a lot of biological questions about dysfunction in synaptic proteins, which are involved in neurodegenerative diseases,” said Jinyoung Kang, an MIT postdoctoral researcher. Up to now, she said, no tool existed that was capable of effectively visualizing synapses.
The researchers also used their new technique to image the beta-amyloid peptide that forms plaques in the brains of Alzheimer’s patients. Using brain tissue from mice, the researchers found that amyloid-beta forms periodic nanoclusters, which had not been seen before. These clusters of amyloid-beta also include potassium channels. Additionally, the researchers found amyloid-beta molecules that formed helical structures along axons.
“In this paper, we don’t speculate as to what that biology might mean, but we show that it exists. That is just one example of the new patterns that we can see,” said Margaret Schroeder, an MIT graduate student who a co-author of the paper.
Boyden and his group are now working with other labs to study cellular structures such as protein aggregates linked to Parkinson’s and other diseases. In other projects, they are studying pathogens that infect cells and molecules that are involved in aging in the brain. Preliminary results from these studies have also revealed novel structures, Boyden said. “Time and time again, you see things that are truly shocking,” he said. “It shows us how much we are missing with classical unexpanded staining.”
The researchers are also working on modifying the technique so that they can image up to 20 proteins at a time. They’re also adapting their process so that it can be used on human tissue samples.
Sarkar and her team, on the other hand, are developing tiny wirelessly powered nanoelectronic devices that could be distributed in the brain. They plan to integrate these devices using expansion revealing. “This can combine the intelligence of nanoelectronics with the nanoscopy prowess of expansion technology, for an integrated functional and structural understanding of the brain,” Sarkar said.
The research was published in Nature Biomedical Engineering (www.doi.org/10.1038/s41551-022-00912-3).