Bio-optics is challenging optical engineers, optical software developers, and medical and molecular scientists to find technological advances that will result in applications that contribute to a healthier world. New design software is helping them meet that challenge.
Michael Stevenson, Breault Research Organization Inc.,
and Steven R. Auger, bioDevice Partners
The fields of genomics and drug discovery would not exist as we know them without the optical systems that enable DNA sequencers, gel imagers, polymerase chain reaction machines, microarray imagers and microfluidics systems to produce the data that are leading to the next generation of medical breakthroughs. Optical technologies enable surgical devices, imaging systems, cancer detection and treatment systems, lab-on-a-chip devices for clinical diagnostics, patient care devices such as glucose monitors and a growing number of other devices.
Optical components also are used in bioterrorism agent detectors, bacterial growth-monitoring systems, and air- and water-quality assessment instruments.
One obstacle to the development of these tools is that physicians, molecular biologists and optical engineers — all highly skilled and knowledgeable of the technologies within their respective fields — do not often interact. They have different lexicons, and they publish in different journals. Greater interaction is necessary as interests and scientific issues converge.
Even as engineers and researchers in disparate fields begin working together, bio-optical systems are some of the most challenging to design because the biological component usually cannot be “engineered” to a specification. Biological systems are intrinsically unique. The optical engineer must take into account sample variation and must thoroughly understand the optical behavior in a “typical” sample. In medical applications, this means engineering systems around the characterizations of fluids or layers of tissue that diffract, scatter or absorb light or that may be damaged by high-energy densities.
Similarly, genomics, bioterrorism agent detection and medical diagnostic systems frequently use fluorophores or luminescent labels that produce optical signals at the lower limit of detection of even the best detectors. Taking measurements can cause the signals to degrade over time through a process called photobleaching, so it must be done quickly. Furthermore, these low signals must be distinguished from the very high excitation light powers and from the background fluorescence produced by the sample vessel, mounting substrate, windows and other molecules in the biological matrix.
To add to these difficult design needs, many applications require very high — frequently, diffraction-limited — resolution and system miniaturization. High resolution allows researchers to observe phenomena on the scale of the microscopic biology, as in cancer cell analysis, or to perform many experiments concurrently, as in lab-on-a-chip systems and microarrays. Miniaturization allows physicians to place a probe or even the entire bio-optical system comfortably inside the patient.
Fortunately, advanced optical-modeling software has been and continues to be developed for the simulation, design and optimization of bio-optical systems. There is one caveat: Besides a state-of-the-art program, extensive background and training is required to produce accurate results with the software application. In any optical project, the engineer must know which optical phenomena to simulate, how to properly model the system, how to ensure manufacturability in the finished product, and what answer to expect from a virtual simulation. The added complexity of bio-optical systems makes their accurate modeling and performance interpretation particularly difficult.
Bio-optical design
For example, when designing a microtiter-plate-based fluorescence imaging system, an optical engineer first must work with a molecular biologist to determine the properties of the biological and physical aspects of the system. Understanding these properties enables the designer to make the appropriate hardware decisions to ensure performance and reliability as well as to offer a cost-effective product.
For the plate reader, key properties of the fluorescent probes to be conveyed by the life scientist to the instrumentation designer must include absorption and emission wavelengths, which affect decisions regarding the optical filter, light source and detector. The designer also must know the sample’s molar absorptivity, quantum efficiency and photobleaching sensitivities, which limit light source power and affect decisions regarding the numerical aperture of the collection lens and the selection of the detector.
The microtiter well dimensions dictate the required optical resolution and collection optics design, and the minimum and maximum number of fluorescent probes per well dictate the components of the optical system that have an effect on sensitivity and dynamic range.
The engineer also must know the properties of the microtiter plate and of any lids over the plate, such as autofluorescence, scattering, index of refraction, thickness and overall size.
As can be seen from this noncomprehensive list, an instrumentation designer experienced with biological systems must know which questions to ask of the molecular biologist.
Once all of this preliminary data is compiled and a system concept outlined, the designer is ready to begin working with optical software packages to simulate the fluorescent plate reader. The engineer creates an initial model of the proposed system to determine the order of magnitude of the signals involved. These simulations establish the anticipated illumination powers required and an acceptable range of detectors. Refining the models and matching these requirements to the available tools and components enable the optical engineer to begin the design and optimization process.
Software packages allow the designer to use existing models of light source output profiles and to couple them to custom optical elements such as reflectors, lenses, fiber optics and light pipes to transmit and shape light. Within the software package, illumination optics are optimized to provide appropriate energy density, throughput and uniformity to each microtiter well. The volume absorption and the resulting fluorescence output from solution samples in the microtiter-plate wells are evaluated.
Once the illumination and output profiles have been determined, optical software is used to design an appropriate fluorescence collection lens and to optimize its numerical aperture, throughput and resolution. Next, stray light and ghost image analyses are performed. These analyses allow the system to be modified to minimize excitation light that might impinge on the detector, such as ghost images of the microtiter-plate wells and scatter from nearby objects, including well walls, lids and support structures. Finally, a working prototype is fabricated and its performance compared with the virtual model.
Today's tools
The design challenges of bio-optical systems can require a wide range of optical software programs, including sequential and nonsequential ray-tracing programs.
Sequential programs trace rays through optical systems, intersecting optical elements in a predefined order. They generally are thought of as lens design programs, and they are of great value to designers during the imaging-system creation and optimization phases.
Nonsequential programs trace rays through optical systems without any predefined order. They are most useful for complex optical design projects involving stray light, difficult illumination goals, coherent optical effects, polarization and scattering.
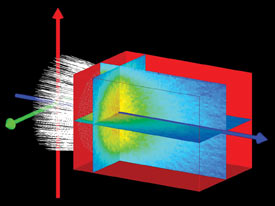
Figure 1. Volume scattering of light is a concern in many bio-optical systems, especially those involving interactions of light with tissue or with blood. Optical software programs can simulate volume scattering using a voxels method.
Light scattering
Light scattering is a key concern in most bio-optical systems, particularly in those attempting to model fluorescence or to simulate light/tissue interactions (Figures 1 and 2). Designers interested in software for bio-optical simulations should thoroughly research the available programs to ensure that the chosen product has the ability and accuracy necessary for the problem or problems posed. In some cases, designers use multiple software packages to fulfill a variety of requirements.
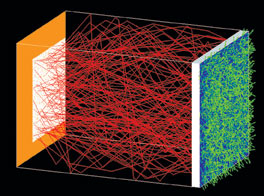
Figure 2. A common motif in the simulation of a bio-optical system combines volume-scattering and fluorescence modeling. Fluorescence in transmission through a volume-scattering medium may be simulated by re-emission of lightthat is scattered and partially absorbed in a voxel volume.
Even with the advanced software tools available to perform the calculations, knowledge of optical engineering is best learned as part of a comprehensive curriculum, beginning with the basics. A core understanding of optics cannot be developed overnight. Researchers and physicians developing products that use optics as enabling technology must consider both the complexity of the optical components involved and the time available to become comfortable with a powerful, yet complex, engineering tool such as optical modeling software.
If the design challenges are not fully understood or the time to come up to speed on an optical software program cannot be invested, outsourcing the work to an experienced optical engineering consultancy is the better option.
The need for optical engineers in the aerospace, automotive, consumer electronics, defense, display, semiconductor and telecommunications industries has resulted in a shortage. Bio-optics experts are even more difficult to find. But optical engineers are working to simulate more of the physics of bio-optical systems in software, which may add functions as well as increase ease of use. Bio-optical projects also will increasingly interest optical engineers, as physicians and bioresearchers seek to harness the full potential of light in their devices.
The key to progress will be education on all fronts: detailed training for nonoptical engineers who wish to go it alone, and background information for optical engineers and software developers being asked to develop state-of-the-art tools and devices for the biotechnology market. With the right education, experience and tool kit, optical software can be used to simulate and design bio-optical and biophotonic systems.
Meet the authors
Michael Stevenson is marketing director at Breault Research Organization Inc. in Tucson, Ariz.; e-mail: [email protected].
Steven R. Auger is the founder and principal consultant at bioDevice Partners in Jamestown, R.I.; e-mail: [email protected].