Long valued for monitoring blood flow, a smaller form factor for laser speckle imaging is being developed to monitor health while overcoming artifacts inherent in mobile readings.
Francesca Bonetta-Misteli and Christine O’Brien, Washington University in St. Louis
Speckle contrast measurements are greatly affected by the
movement of the imaging system because it increases the blurring in images.
While laser speckle contrast imaging (LSCI) has historically been confined to a bulky benchtop configuration, multiple research teams have recently developed hand-held and wearable laser speckle systems. For these systems to be useful in a clinical setting, hand-held devices must be lightweight and compact enough to easily lift and move around while wearable users must be able to function normally. This requires careful selection and characterization of camera and laser components; for untethered systems, this requires small and inexpensive computation and battery modules, and intentional hardware design.

A wearable laser speckle contrast imaging (LSCI) device from Washington University in St. Louis on a human arm, recording data during arm occlusion. Courtesy of O’Brien Lab/Washington University in St. Louis.
Although more clinically practical, hand-held systems can introduce motion artifacts in images due to unpreventable movements by the operator. Speckle contrast measurements are greatly affected by movement of the imaging system because it increases the blurring in images. Wearable systems minimize motion artifact as the device moves with the subject but are more spatially confined to fit all necessary components into a comfortable form factor. The use of smaller, inexpensive components allows for the possibility of wearable systems but can degrade signal quality. For hand-held and wearable LSCI systems to be successful, it is essential to develop innovative approaches to increase signal quality under component constraints and to provide motion artifact detection, correction, and/or prevention.
The basics
LSCI is an optical technique that uses a coherent light source and a camera detector to monitor blood flow in vivo (Figure 1). As coherent light interacts with human tissue, it is scattered, and some of the scattered light is captured by a camera detector. On the camera detector, the scattered light appears as a random interference pattern, called laser speckle. Depending on tissue dynamics, the speckle pattern may change over time. If scatterers in the tissue (such as red blood cells) are quickly moving, the speckle pattern will change rapidly, whereas static scatterers (such as skin cells) will result in a non-changing speckle pattern.

Figure 1. An example of a raw speckle image (left) and speckle contrast image (right). Adapted with permission from Reference 1.
Fluctuations in the laser speckle pattern that occur over the exposure time of a camera create blurring in images. The degree of blur is related to the velocity of dynamic scatterers in the substance being imaged. The blurring of the laser speckle pattern is quantified through calculating the speckle contrast value (K), which is defined by the following formula:

where σ(I) is the standard deviation of pixel intensity and <I> is the mean of pixel intensity. Images can be processed spatially by sliding an nxn pixel window across an image and calculating K for each window, creating a speckle contrast image. Images can alternatively be processed temporally, by calculating K using the same pixel index from n frames. Spatial processing results in high temporal resolution, while temporal processing results in high spatial resolution.
The 2D images of speckle contrast can be used in several ways for various applications. From the speckle contrast, a value directly proportional to blood flow — termed laser speckle flow index (LSFI) — can be computed using the following equation:
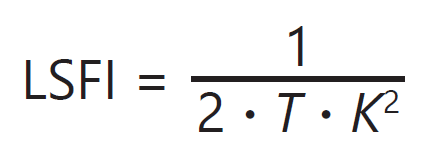
Hand-held devices are
particularly ideal in hard-to-
reach areas that may
be difficult to image using
stationary systems.
where T is the exposure time of the camera. If sequential frames are captured, then 2D images of LSFI can be used to monitor blood flow in space or over time. The mean LSFI value measured for individual images or regions of interest within images in a set of sequential frames has also been used as a metric for monitoring average blood flow as a function of time. This can be useful when examining changes in blood flow, such as those caused by stroke, traumatic injury, or hemorrhage over time and in response to certain hemodynamic stimuli.
Toward compact LSCI systems
Typical LSCI systems are large and expensive benchtop systems that produce 2D images of speckle contrast. The most common research applications of LSCI have been in brain blood flow mapping and ophthalmology. There are commercially available systems; however, clinical use of LSCI systems is not widespread, which is likely due in part to high cost and bulky form factors. Most commercial and even research-grade systems use expensive, highly coherent lasers and accompanying current drivers and temperature control units, along with expensive, megapixel, high-sensitivity cameras. Processing is performed on laptops or desktop computers. In recent years, there has been movement toward developing compact, hand-held, and even wearable LSCI sensors. This new wave of devices has a large potential to overcome the bulk and cost barriers to clinical translation faced by existing large and expensive benchtop systems.
Hand-held LSCI systems
A study published in 2019 directly compared pulsatile speckle signal computed from measurements of a human forearm performed by both a hand-held and stationary device2. When using the hand-held device, the pulsatile speckle contrast signal, produced by the increases and decreases in blood flow that occur with every heartbeat, produced a smaller dynamic range compared to the measurements made with the device when positioned in a stationary system. While the hand-held system had a lower footprint and cost, without implementing methods to account for motion artifacts, the signal produced by the hand-held system performed more poorly compared to benchtop systems.
One approach to preventing motion artifacts from interfering with speckle measurements is to use fiducial markers such as reflectance cards, medical tape, or other visual markers3,4. Markers can be used to align a sequence of captured speckle images or to quantify the magnitude and direction of movement between speckle frames. In addition, movement vectors can be calculated using electromagnetic-tracking or other movement-tracking techniques. These techniques can be used to define the imaging system motion and then custom algorithms can be developed to correct for movement and produce accurate speckle contrast images.
Correcting for movement is complex because the effect of an imaging system’s movement on the speckle measurement is not an absolute measure of flow speed and is dependent on the optical properties of the object being measured. Movement artifacts in speckle images cause a decrease in the measured speckle contrast, as shown in an in vitro flow model. When objects with lower scattering coefficients are imaged, motion artifacts have a more significant effect on the measured signal2. This suggests that to correct for motion artifacts, one would need to calculate the movement vectors of the imaging system as well as the optical properties of the object being examined.
Another approach to solving the motion artifact problem integrates innovation in hardware to design devices that prevent motion artifacts from affecting their signal. In 2019, a team of researchers from University of California (UC) Irvine developed a hand-held device with a gimbal stabilizer that significantly improved the signal-to-noise ratio of speckle data and increased the number of usable frames captured (Figure 2)3. Hardware innovations, such as the gimbal stabilizer, could be used in conjunction with movement characterization or fiducial marker alignment techniques to further minimize motion artifacts.

Figure 2. A motion-stabilized laser speckle imaging device developed by the lab of Bernard Choi at the University of California (UC) Irvine. This fully assembled device uses a gimbal stabilizer. Adapted with permission from Reference 3.
Wearable LSCI systems
Another approach employed by a number of researchers to overcome motion artifacts is to have LSCI devices that are wearable and make direct contact with the object being measured. If the imaging device is secured directly on the subject, such as on the head or on a limb, the risk and effect of motion artifacts are greatly decreased because the device moves with the subject5. The drawback of this method is that it does not allow for imaging of large areas of tissue or rapid movement between different anatomic locations, but it can be highly useful in many clinical applications, particularly in the monitoring of general cardiovascular health or in anesthesiology.
In 2016, a team of researchers from the University of Kentucky published work related to their design and evaluation of the performance of a novel speckle system that captured speckle images when in direct contact with human tissue6. Shortly after publishing this work, groups from UC Irvine, Washington University in St. Louis, and others developed compact LSCI devices that capture speckle while being in direct contact with the subjects being imaged. From UC Irvine in 2018, Michael Ghijsen, among others, developed an LSCI device as a finger clip, similar to the form factor of a pulse oximeter5. The device was configured in transmission mode with the laser source on the top of the finger, and the camera detector collected light that was transmitted through to the bottom of the finger.
Typically, LSCI is performed in reflectance mode with the laser and camera sensor on the same side of the object being measured. By configuring the device in transmission mode, it could fit easily into a finger cuff and be in direct contact with the object being measured. To fit into a wearable, the laser source was a small diode and there were no optical fibers.
This transmission mode device can collect high-quality pulsatile speckle signals from human subjects. There were no apparent motion artifacts in the recorded data because the device hardware prevented the imaging sensor from moving with respect to the finger during recordings. The device was also used to measure a fluid flow phantom across a range of flow rates, which resulted in a linear correlation between fluid flow rate and LSFI signal (R2 = 0.98)5. This linear relationship indicated that the device can distinguish between different fluid flow rates and provides a level of accuracy similar to larger high-cost benchtop systems.
A wireless and wearable LSCI system was similarly developed by the research team at Washington University in St. Louis in 20237. To the author’s knowledge, this device is the first wireless and wearable LSCI system. The benefit of being completely wireless and untethered is that motion artifacts can be further minimized, even outside of a highly controlled laboratory setting; the system can be securely fixed to a subject and will not be disrupted by a moving tether. Additionally, the subject can move freely, which supports continuous monitoring over hours and days.
This device records speckle signal in reflectance mode, like most LSCI systems. However, it is configured with a short optical train that consists of a single fixed-focus lens on the camera sensor, a second identical lens sitting inverted on the surface of the first, and a small optical window on the surface of the second lens. This train allows the camera to be in focus with the surface contacting the device, such as patient skin (Figure 3).

Figure 3. A full wrist-worn wearable device that was designed by the lab of Christine O’Brien at Washinton University in St. Louis (a). The design for a laser and camera system for imaging blood flow (b). Courtesy of O’Brien Lab/Washington University in St. Louis.
The device is the first of its kind to be made wireless; the laser and camera are controlled by a Raspberry Pi Zero computer connected to custom circuitry and a small lithium polymer battery. The device is Bluetooth-enabled, so data can be collected and transmitted to a receiving device for near real-time data visualization. Again, using a completely untethered approach is an optimal method for minimizing motion artifacts because the system can be attached securely to a subject and will not be disrupted by a moving tether. Furthermore, the wireless device allows for continuous monitoring for days and even weeks, which opens up a host of new clinical applications, such as monitoring peripheral vascular diseases and burn wounds from the bedside.
Data is captured on the device and down-sampled frames are processed and then sent via Bluetooth to a receiving device (opening image). Because processing speckle images is computationally expensive, a fast-processing algorithm was developed for near real-time visualization of data. As LSCI device hardware becomes wireless and Bluetooth data transmission is required, fast-processing algorithms will become increasingly important for true real-time (minimum 30 fps) data visualization and frame-by-frame processing.
Low-cost LSCI systems
There have been advancements in the development of portable and low-cost LSCI systems during the past five years, which
have the potential to yield widespread adoption within clinical settings.
Benchtop LSCI systems are typically expensive because highly coherent laser sources often require constant current drivers and intricate temperature regulation systems. Similar to expensive laser systems, CMOS cameras with high sensitivity, resolution, and speed can cost hundreds or even thousands of dollars. Some groups have successfully used smaller and cheaper laser diodes with minimal drivers or temperature control, and smaller, lower-resolution, inexpensive cameras. Using these components, expectedly, will produce lower-quality speckle signal, but they can still yield sufficiently high contrast and signal-to-noise ratio images that can meet the needs of many medical applications. The performance of systems can be objectively assessed using a flow phantom model, in which speckle images are captured when imaging fluid flowing through a channel at different velocities. The O’Brien group captured high-quality speckle images using their integrated low-cost wearable LSCI system and compared the performance of the system while using a low-cost laser diode and high-cost benchtop laser in a flow phantom model (Figure 4).
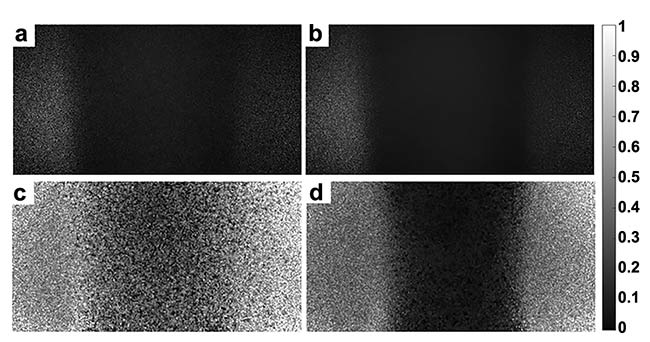
Figure 4. Captured images from the low-cost wearable and wireless laser speckle contrast imaging (LSCI) device from the O’Brien Lab. A raw speckle image in a low (a) and high (b) flow phantom tube. A speckle contrast image in a low (c) and high (d) flow phantom tube. Courtesy of O’Brien Lab/Washington University in St. Louis.
The low-cost system cost about $100 to produce, using mostly off-the-shelf components, and the high-cost benchtop laser was ~100× more expensive. The effects of the two laser sources were directly compared by examining the linear relationship produced between the measured flow index and fluid velocity when measuring speckle from a flow phantom, with different fluid velocities pumped through the phantom with a syringe pump. The results of this experiment show that both the high-cost and low-cost lasers produced linear correlations between fluid velocity and flow index (Rlow-cost = 1 and Rhigh-cost = 0.99).
Notably, the high-cost system produced a wider range of flow index values and had a larger slope of the linear regression curve. This means that for the same difference in flow rates, the high-cost system produced a more significant change in speckle contrast value and flow index. Although the high-cost system performs with higher sensitivity, the low-cost system could still accurately distinguish between flow rates. With a continued push to make LSCI systems cheaper and more accessible, it will be important to continue to determine methods for maintaining or increasing signal-to-noise ratio while using low-cost and smaller components, an effort being pursued by groups at Washington University in St. Louis, the University of Kentucky, and Interuniversity Microelectronics Centre (IMEC) in the Netherlands.
Looking to the future
There have been major advancements in the development of portable and low-cost LSCI systems during the past 5 years, which have the potential to yield widespread adoption within clinical settings. Hand-held LSCI devices, which create a 2D map of blood flow in tissue, can be used for retinal imaging to diagnose various eye-related conditions, as well as skin imaging to aid the diagnosis of various dermatologic conditions, and monitor burns and healing wounds from surgery, especially surgical flaps, which must quickly restore perfusion after transplant. Hand-held devices are particularly ideal in hard-to-reach areas that may be difficult to image using stationary systems.
Unlike the hand-held devices, wearable systems image much smaller sections of tissue and would not be as useful for creating maps of blood flow in vivo. However, these systems can be used to longitudinally record blood flow at various anatomic sites, including the periphery, head, and neck to monitor for surgical complications and assess brain or cardiovascular health in infants, children, adults, and animals. Wearables can also be used for real-time monitoring of blood loss, which causes vasoconstriction in the hands and forearms, decreasing blood flow. Also, new motion artifact computation and removal algorithms will be necessary for clinical use of hand-held devices, and various research groups are pursuing these goals. Others — such as those at the University of Kentucky and the National Institutes of Health — are continuing efforts toward improved wearable speckle systems for use on humans and in animal models for research purposes.
Meet the authors
Francesca Bonetta-Misteli is a doctoral candidate at Washington University in St. Louis in the O’Brien Lab in the Department of Biomedical Engineering. She received her bachelor’s degree in biomedical engineering from Washington University in St. Louis. She is interested in developing optical technologies and point-of-care diagnostics to solve global and women’s health problems. Her work is focused on the development of a low-cost wearable laser speckle contrast imaging device for monitoring of peripheral perfusion and early detection of postpartum hemorrhage; email: [email protected].
Christine O’Brien is an assistant professor of biomedical engineering at Washington University in St. Louis with a secondary appointment in the Department of Obstetrics and Gynecology. She obtained her doctorate in biomedical engineering at Vanderbilt University and completed postdoctoral training at Washington University School of Medicine in the Department of Radiology. She launched her independent research program with projects focused on the development of novel wearable sensors for the early detection of postpartum hemorrhage and novel strategies for preterm birth detection and investigation; email: [email protected].
References
1. A. Ponticorvo and A.K. Dunn. (2010). How to build a laser speckle contrast imaging (LSCI) system to monitor blood flow. J Vis Exp, Vol. 11, No. 45, p. 2004.
2. A. Chizari et al. (2020). Exploration of movement artefacts in handheld laser speckle contrast perfusion imaging. Biomed Opt Express, Vol. 11, No. 5,
pp. 2352-2365.
3. B. Lertsakdadet et al. (2019). Handheld motion stabilized laser speckle imaging. Biomed Opt Express, Vol. 10, No. 10,
pp. 5149-5158.
4. A. Chizari et al. (2021). Handheld versus mounted laser speckle contrast perfusion imaging demonstrated in psoriasis lesions. Sci Rep, Vol. 11, No. 1, p. 16646.
5. M. Ghijsen et al. (2018). Wearable speckle plethysmography (SPG) for characterizing microvascular flow and resistance. Biomed Opt Express, Vol. 9, No. 8, pp. 3937-3952.
6. C. Huang et al. (2016). Low-cost diffuse speckle contrast flowmeter using small laser diode and bare charge-coupled-device. J Biomed Opt, Vol. 21, No. 8,
p. 80501.
7. F. Bonetta-Misteli et al. (2023). Development and evaluation of a wearable
peripheral vascular compensation sensor in a swine model of hemorrhage. Biomed Opt Express, Vol. 14, No. 10,
pp. 5338-5357.