Advances in interferometry and variations of optical coherence tomography may soon lead to breakthroughs in diagnostics and clinical medicine, including the fields of ophthalmology, surgery, cancer detection, burn management and malaria.
Optical coherence tomography (OCT) began its commercial history in 1996 as a revolutionary technology for imaging retinas to help diagnose eye diseases, including glaucoma, macular degeneration and diabetic retinopathy. Now a mainstay in ophthalmology offices, OCT technology has expanded its reach to numerous other biomedical applications, including cardiology, IVF and oncology.
OCT is a close cousin to ultrasound, which uses sound waves to image through skin and other tissue, whereas OCT uses light waves. The key technology behind OCT is low-coherence interferometry, in which a Michelson-type interferometer measures the scattering, absorption and/or reflection of a broadband light source with short temporal coherence through tissue. The success of OCT in so many biomedical applications is due to the fact that tissue efficiently scatters light in a “biological window” from 800 to 1300 nm. Light in this wavelength range can image tissue to a maximum depth of up to approximately 2 mm, according to Stephen Boppart, professor of electrical and computer engineering at the University of Illinois at Urbana-Champaign in Urbana, Ill.
Recent research and development areas in interferometric techniques are too numerous to list, but hot areas include full-field OCT (FF-OCT), confocal Raman spectroscopic OCT (CRS-OCT) (Figure 1), ultrahigh-resolution OCT (UHR-OCT) and swept-source OCT (SS-OCT) . Combining current technology with these new advances, the OCT market is expected to grow 11.37 percent annually to reach $1.87 billion by 2022, driven by an aging population and the resulting need for faster, better medical diagnostics1.

Figure 1. Researchers are expanding interferometric techniques to improve medical diagnostics. This setup at Duke University combines co-localized confocal Raman spectroscopy and optical coherence tomography (OCT) to better assess burn injuries. Courtesy of Jason Maher/Duke University.
Full-field OCT
Current cancer screening techniques are notorious for over-identification of clinically insignificant cancers, leading to overtreatment and overburdening of patients. Furthermore, tissue processing of biopsies is a time-consuming procedure that can take up to seven days. One area of interferometry leading to success in real-time cancer screening is full-field OCT, which enables pathologists to image tissue in-depth at the cellular level with high resolution and high sensitivity. Claude Boccara, a professor of medical physics at ESPCI Paris Tech in Paris, pioneered FF-OCT and partnered with imaging system developer LLTech SAS, also in Paris, to bring it to fruition.
Unlike most OCT techniques, the FF-OCT approach images across a large area in two dimensions using white light interference microscopy. The elimination of a large depth-of-field enables a wider field to be imaged at depths of hundreds of microns with a resolution of 1 μm, sufficient for resolving details at the cellular level — and 10× more detail than regular OCT.
Boccara and LLTech launched an FF-OCT scanner called the Light-CT for research studies in 2011, and it became available for clinical studies in 2013. The next step was to evaluate the diagnostic accuracy of the scanner for cancer detection. In 2015, the company partnered with biomedical researcher Nicolas Barry Delongchamps at Paris Descartes University and colleagues to identify how well the Light-CT could help identify tumors in ex-vivo kidney and prostate tissue2,3.
In one study, Delongchamps’ team used a Light-CT Scanner to assess the tissue of patients with signs of prostate cancer and found pathologists agreed with an average accuracy of 72 percent on high Gleason score (>3 + 3) diagnoses in blind analyses of the samples. Gleason is a cancer evaluation system that grades cells as normal (1) to very abnormal (5); the highest Gleason score is 5 + 5. Whereas current histology protocol and final diagnosis on typical prostate biopsies can take up to seven days, the new FF-OCT technology can help detect cancer in just a few minutes, helping radiologists to make an accurate diagnosis while the patient waits. As an added bonus, the margins of the biopsy area remain intact for final histology (Figure 2).
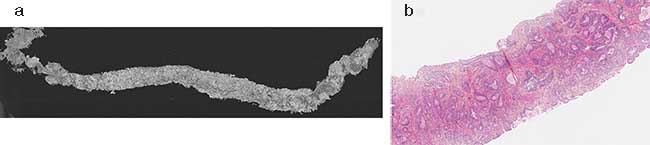
Figure 2. The LLTech Light-CT Scanner uses full-field OCT (FF-OCT) to produce 2D images of prostate cancer biopsies with a transverse resolution of 1.6 µm, depending on depth. The technique produces a single black and white image in digital imaging and communications in medicine (DICOM) format of a whole prostate biopsy sample (a). A zoomed-in section in pink, measuring approximately 1 mm, reveals aggregated tumor glands with a cancer diagnosis of Gleason 3 + 3 (b). The technique can aid in faster cancer detection. Courtesy of Eugénie Dalimier/LLTech SAS.
“LLTech is translating a novel optical technology, developed in a research institute, into a powerful real-time diagnosis tool,” said Eugénie Dalimier, co-author on the prostate study and product development head at LLTech Imaging. “It has potential to dramatically improve cancer surgery and cancer diagnosis, with high impact for the patient and the health-care system.”
Boccarra and colleagues plan to continue improvements in resolution and depth-of-field via a new approach called dynamic FF-OCT (D-FF-OCT), which acquires images of subcellular behavior at short time-scales of less than 10 ms. In this approach, metabolic activity provides the additional contrast, in situ, at the subcellular scale4.
Spectroscopy and OCT
Many OCT techniques capture only the one dimension of the information carried by light — the amplitude, which gives axial depth information. But interferometry can offer up access to other kinds of information. Researchers at Duke University in Durham, N.C., are using several techniques to capture the spectroscopic information in OCT to help in the diagnosis of disease. Adam Wax, the Theodore Kennedy Professor of Biomedical Engineering at Duke, and colleagues have used spectroscopic OCT (S-OCT) to measure wavelength-dependent absorption and scattering to help assess burn injuries.
Currently, evaluating burns depends upon subjective visual inspection, which is limited in accuracy. Quantitative measurement of burn wounds could help better predict whether a wound will heal within a few weeks or require surgery. With S-OCT, doctors can make a more accurate assessment of the depth of burn injuries to skin in vivo (Figure 3).
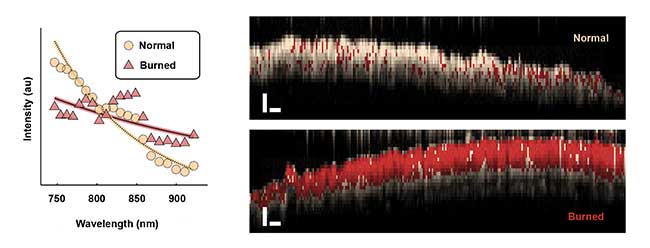
Figure 3. Average spectra backscattered from normal and burned tissues (left) are extracted with spectroscopic OCT. The plot reveals a difference in overall slope as well as oscillatory features in the burned tissue spectrum. Spectroscopic OCT images of normal and burned tissue (right) in live mice. A logistic regression model is used to classify each pixel as normal (white) or burned (red; scale bars = 250 µm). Courtesy of Jason Maher/Duke University.
The Duke study used a spectral-domain OCT system from Wasatch Photonics Inc. in Durham, N.C., centered at 850 nm to image the skin of live mice with axial and lateral resolutions of 2.1 µm and 8 µm, respectively5. The team then processed the data via custom MATLAB software using short-time Fourier transform and dual window methods. Consequently, the technique acquired conventional intensity-based depth information as well as cellular detail of necrosis that enabled classification of burn severity.
In another study, Wax and Duke University professor of biomedical engineering David Katz directed an experiment combining two complementary optical techniques — confocal Raman spectroscopy and optical coherence tomography (CRS-OCT) in a single instrument6 (Figure 4). This technology allows separate acquisition of inelastic Raman scattering data and cross-sectional OCT images from microscopic tissue regions with sub-100-micron spatial resolution, which reveals molecular information that scientists can then use to quantify concentration profiles of topically applied drugs. Using CRS-OCT, the Duke team was able to detect and measure depth-resolved, physiologically relevant concentrations in epithelial and stromal tissue of the drug Tenofovir, a microbicide used to prevent HIV transmission.
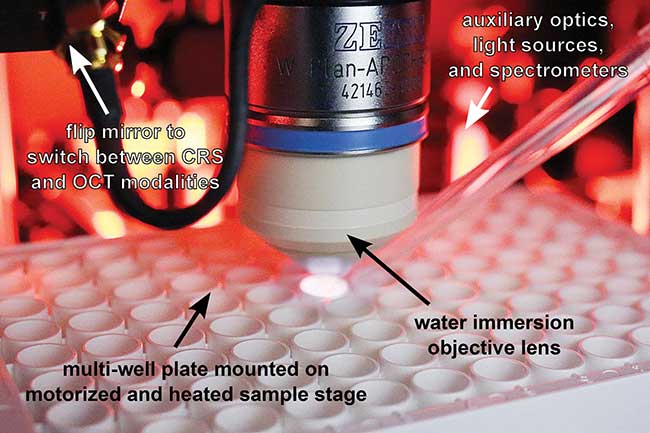
Figure 4. The confocal Raman spectroscopy and optical coherence tomography (CRS-OCT) instrument can obtain co-localized, cross-sectional OCT images and chemically specific Raman spectra with sub-100-micron spatial resolution, enabling the study of ex-vivo drug delivery. Courtesy of Jason Maher/Duke University.
Other upcoming projects for the Duke team include the use of interferometric digital holography to detect malaria and sickle-cell anemia in red blood cells, and the results of a clinical pilot study in real-time diagnosis of cervical precancer using angle-resolved low-coherence interferometry (a/LCI). Wax hopes that positive results from the study may lead to eventual commercialization of the technology.
“Creating a biomedical device that is useful to physicians offers us the opportunity to indirectly improve patient care and have an effect on many people’s lives. This gives our work a greater impact,” said Wax.
Handheld OCT
In addition to amplitude, OCT systems also capture phase data, which isn’t always used, according to Boppart, at the University of Illinois.
“Phase-based imaging techniques are a growing area of study in biomed imaging,” said Boppart, whose group uses amplitude and phase information to computationally improve OCT systems.
When a beam focuses into an object or tissue, the focal point is small. Areas above or below will be out of focus. Using a technique called interferometric synthetic aperture microscopy (ISAM), Boppart and colleagues were able to reconstruct the phase information of the blurry part of the target and image it with the same resolution achieved at the focal plane. But aberrations remained a challenge.
In uncorrected OCT imaging, optical aberrations in the eye restrict the imaging resolution of the retina to no better than 10 or 15 µm. Boppart and colleagues created an automated computational aberration correction and a phase7. This computational approach works like conventional hardware-based adaptive optics to enable high-resolution volumetric imaging but with less complexity and cost.
These advances resulted in two spinoff companies now commercializing compact, real-time imaging and diagnostic OCT systems. Diagnostic Photonics Inc. in Chicago has recently obtained FDA approval for high-performance handheld systems designed to help breast cancer surgeons improve resolution of tumor margins and remove all tumor cells. And PhotoniCare Inc. in Champaign, Ill., with Boppart as chief medical officer, expects FDA approval later in 2016 of a similar device for visualization behind the eardrum for use in primary care offices (Figure 5). To enable lower-cost handheld scanners, Boppart’s team is working on eliminating the costly microelectromechanical systems (MEMS) transverse scanner and replacing it with a movement tracker like that in an optical mouse8. Commercialization of such low-cost handheld OCT scanners may happen in the next five years, according to Boppart.
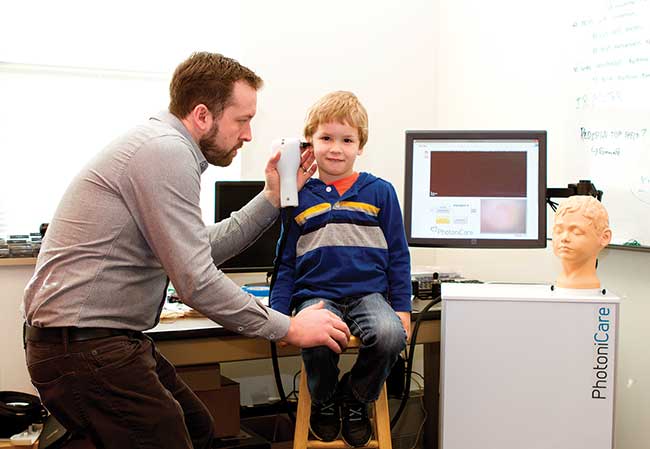
Figure 5. A handheld OCT imager allows visualization through tissue for better diagnoses and treatment of issues like middle ear infections and breast cancer. Courtesy of Stephen Boppart/PhotoniCare Inc., Community Concierge Magazine.
“All parents can relate to the need for children to have earlier and more accurate diagnoses of ear infections,” said Boppart. “The same can be said about breast cancer. These developments truly resonate with everyone.”
References
1. Stratistics Market Research Consulting (September 2015). Global optical coherence tomography market outlook (2014-2022), online.
2. J. Lopater et al. (June 2015). Real-time cancer diagnosis during prostate biopsy: ex vivo evaluation of full-field optical coherence tomography (FF-OCT) imaging on biopsy cores. World J Urol, (doi: 10.1007/s00345-015-1620-6).
3. M. Jain et al. (September 2015). Rapid evaluation of fresh ex vivo kidney tissue with full-field optical coherence tomography. J Pathol Inform, Vol. 6, Issue 53 (doi: 10.4103/2153-3539.166014).
4. C. Apelian et al. (submitted Jan. 6, 2016). Dynamic full-field optical coherence tomography: subcellular metabolic contrast revealed in tissues by temporal analysis of interferometric signals. arXiv: 1601.01208.
5. Y. Zhao et al. (2015). Evaluation of burn severity in vivo in a mouse model using spectroscopic optical coherence tomography. Biomed Opt Expr, Vol. 6, Issue 9, p. 3339 (doi: 10.1364/BOE.6.003339).
6. J. Maher et al. (June 2015). Co-localized confocal Raman spectroscopy and optical coherence tomography (CRS-OCT) for depth-resolved analyte detection in tissue. Biomed Opt Expr, Vol. 6, Issue 6, p. 2022 (doi: 10.1364/BOE.6.002022).
7. N. Shemonski et al. (July 2015). Computational high-resolution optical imaging of the living human retina. N Photon, online, Vol. 9 (doi: 10.1038/nphoton.2015.102).
8. P. Pande et al. (November 2015). Sensor-based technique for manually scanned hand-held optical coherence tomography imaging. J Sens, Vol. 2016 (doi: 10.1155/2016/8154809).