Power generation from artificial photosynthesis is a green dream beginning to grow tall.
On a warm summer afternoon, you may find yourself on a leisurely walk through bucolic meadows or toiling under the glare of the sun because the lawn just won’t mow itself, but why not let the grass do some work and earn its keep?
Grass, as does all green plant life, gets the energy it needs to thrive and reproduce by using sunlight to convert chlorophyll, carbon dioxide and water into sugar (giving off oxygen as a by-product). Ever since this light-driven mechanism was first elucidated, scientists and engineers have wondered about the possibility of replicating it to power homes, cars or gadgets.
Finding out what makes plants tick was one thing, but redesigning the process using man-made materials has not been easy. Furthermore, the hoped-for result isn’t food at all but rather hydrogen fuel or electrons that can be either channeled into a storage battery or used to power a device directly. No matter which approach is taken, the name of the game is “artificial photosynthesis.”
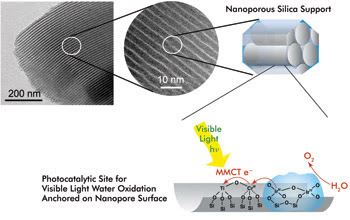
Artificial photosynthesis depends on efficient charge separation and transport to get the most value from solar photons. Besides effective chlorophyll substitutes, a photosynthetic system must employ a substrate material that helps capture and shuttle light. At Lawrence Berkeley National Laboratory, specialized light absorbers act as visible-light electron pumps for driving water oxidation or CO2 reduction. Three-dimensional nanoporous supports arrange the photocatalytic units. Courtesy of Heinz Frei.
According to Hans Desilvestro, chief scientist at Dyesol in Queanbeyan, Australia, the Earth receives nearly 100,000 times more energy from the sun than is required worldwide every year. In the US, energy consumption is currently 15 to 25 TW annually, but even as that figure rises to 45 to 50 TW by century’s end, it pales next to the 2200 TW available from solar.
The most common approach to achieve artificial photosynthesis – and the one taken by Dyesol – is to develop a photo-reactive dye that takes the place of chlorophyll. As with the natural material it tries to mimic, the dye must include molecules that capture photons, then transfer the electrons kicked loose by the light’s energy. It is not enough to duplicate the solar conversion ability of chlorophyll, however; plants convert only a fraction of the energy provided by the sun that people would need.
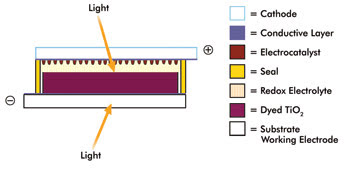
A schematic diagram of a dye-sensitized solar cell highlights the key components. Courtesy of Dyesol.
Dyesol’s efforts are toward the development of a solar cell whose main constituents are titanium dioxide, a photoactive chlorophyll analogue and an iodide-based reduction-oxidation (redox) compound.
The basic Dyesol cell structure starts with a working electrode made of a substrate coated with a <20-μm film of nanoparticulate TiO2. Atop this base lies a single-molecule layer of a photoactive dye such as a ruthenium-based compound, followed by a sealant gasket and a redox electrolyte layer containing iodide and tri-iodide. The cell is completed with a counterelectrode made of a conductive substrate coated with a thin layer of catalyst material. Desilvestro said that the substrates may be made of conductive glass, plastic or metal foil, although at least one must be transparent. Light entering through the transparent substrate causes charge separation in the electrolyte, and that charge is then collected, stored and applied to power a device.
Dyesol uses a nanoporous form of TiO2 for two reasons: First, the scale and porosity of the material improve its light-gathering ability; second, the material can be painted onto the substrate or even screen-printed to create decorative designs.
Ultimately, the company sees so-called dye-sensitized panels as overtaking even photovoltaic solar technology.
“It takes three to four years in sunny climates – eight years or longer in areas such as Germany or northern Europe – for a traditional photovoltaic panel to produce the amount of energy which went into its production,” Desilvestro said. “The corresponding energy payback period for dye-sensitized panels is only two to six months under the same conditions.”
Splitting water
Energetically beating electrons out of a chlorophyll substitute is not the only way to think of artificial photosynthesis. The other major research approach is to use the sun’s energy to separate water into its hydrogen and oxygen components. The hydrogen, kept in its ionic form, would then be collected for use in fuel cells or similar power-generating technology.
Scientists at the Tempe campus of Arizona State University’s newly minted Energy Frontier Research Center for Bio-Inspired Solar Fuel Production are working on the hydrogen fuel approach.
One of the most challenging hurdles in artificial photosynthesis is the formation of molecular reaction centers – constructs that replicate the plant enzymes that capture sunlight and use its energy to create fuel for the plant. Courtesy of Devens Gust, Arizona State University.
“[Artificial photosynthesis] incorporates the basic science of photosynthesis into technological systems that will be optimized to produce energy in forms useful to humans,” said Devens Gust, a chemistry professor and director of the solar fuel research center.
A key concept being addressed by Gust and his colleagues is the re-creation of the reaction centers in photosynthesizing plants – the molecules that convert light energy into chemical energy. And, again, replication is not enough; man-made reaction centers must improve on the original.
Gust said that most of the sunlight used in natural photosynthesis is not absorbed by a plant’s reaction centers; rather, the light is gathered by simpler molecular “antennas” that then send the excitation energy to the reaction centers. Furthermore, the antennas help regulate the photosynthetic process and prevent damage from too much light.
Plant life requires fuel, but far less than people and their electrical appliances. Natural photosynthesis converts light into energy at a fraction of a percent efficiency. Researchers are aiming at 10 percent efficiency as a start. To achieve a higher rate, Gust and his associates Thomas A. and Ana L. Moore are testing a number of potential biomimetic candidates, including carotenoids, which are readily available organic pigments.
Out of the blue
Another potential candidate for helping the artificial photosynthesis process is cobalt. At Lawrence Berkeley National Laboratory in California, Heinz Frei and his colleague Feng Jiao found that nanometer-scale crystals of cobalt oxide (Co3O4) efficiently separate oxygen from water molecules.
Plants use manganese-infused enzymes to catalyze the water-splitting process, but Frei’s group wanted a material that also was water-soluble. Iridium was a strong option, but it is very rare and thus too expensive for large-scale use. Cobalt also was a candidate, but the researchers first tried micron-scale, which turned out to be too inefficient and slow to react to light. However, when they used nanoscale Co3O4 crystals, efficiency spiked by 1600 percent. The best performers were 8 × 50-nm cobalt clusters.
An ANSER to the question
Increasing the efficiency of solar conversion is the overwhelming theme to artificial photosynthesis projects across the globe. The drive for more power per photon is driving collaborative efforts as well, such as ANSER, the Argonne-Northwestern Solar Energy Research Center.
ANSER combines the efforts of scientists at Northwestern University and at Argonne National Laboratory, both in Illinois, as well as at Yale University, at the University of Illinois at Urbana-Champaign and at the University of Chicago. Its impetus is to explore solar power as a means to provide clean sources of energy to fulfill a growing demand.
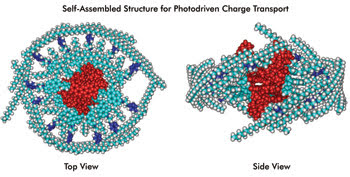
Self-assembling electron donor-acceptor building blocks form protein-size charge-transport systems that could be used to create artificial photosynthesis systems for fuel or flexible organic solar cells. Courtesy of Michael Wasielewski, Northwestern University.
“We hope that the fundamental science and technology that we are currently developing will result in widespread use of cheap solar-driven energy technologies based on artificial photosynthesis,” said Michael R. Wasielewski, chemistry professor at Northwestern and director of ANSER. “The turning point will occur when these technologies are 10 percent efficient at price points that are below today’s energy supply costs from competitive technologies.”
Investigators affiliated with ANSER are looking into both direct generation of electricity and production of hydrogen or other fuels using artificial photosynthetic methods. As with similar programs from Europe to Australia, the main mission is to find the right sustainable, easy-to-build structures that at first mimic, then improve upon, the way greensward has fueled itself for millennia.