Wide-area apertures provide enhancements over slit-based systems.
Dr. Mike Fuller, Dr. Prasant Potuluri and Michael Sullivan, Centice Corp.
The new and rapidly expanding
field of nanoscale medical diagnostics requires the development of advanced sensor
technologies to provide the selectivity and sensitivity needed to measure concentrations
of target biomarker molecules at subnanomolar concentrations. These demands for
higher sensitivity and, hence, lower detection limits have driven the development
of novel approaches to molecular detection.
Over the past 20 years, there have been only minor
advancements in the technologies used in instruments designed for molecular spectroscopy,
including those based on the Raman effect. Commonly, slit or pinhole apertures
spatially define the light entering the section of a spectrometer that separates
and detects wavelengths. In modern instruments, scanning monochromators largely
have been replaced with fixed dispersion gratings coupled with either linear or
two-dimensional CCD array detectors.
To achieve useful spectral resolution,
the entrance aperture is usually fixed at around 50 to 100 μm. This small entrance
aperture severely limits light-collection efficiency and, as a result, sensitivity.
Recently, analytical spectrometers using multimodal multiplex spectroscopy technology
have been introduced. These spectrometers employ wide-area, binary-encoded apertures
and computational transforms to greatly improve sensitivity without compromising
spectral resolution.
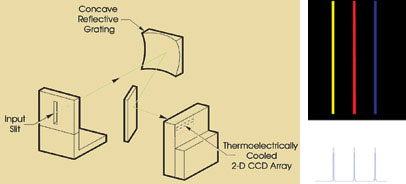
Figure 1. A schematic illustrates the optical
layout of a typical slit-based spectrometer (left), accompanied by a typical CCD
detector image and spectrum that it can record (right).
In a slit-based dispersive instrument,
the spectral width of a resolution element is directly proportional to the width
of the slit (Figure 1). This relationship provides a challenge to spectroscopy in
real-world conditions. To achieve a reasonable spectral resolution, the input slit
must be narrow. However, real-world light sources are so diffuse in nature that
only a small percentage of the total light can enter the narrow slit of the spectrometer.
If the light source is weak, then the detector can be so starved of photons that
no usable spectral measurement is possible. In these traditional designs, there
is an inherent trade-off between resolution and light throughput.
In contrast, an instrument based on
multimodal multiplex spectroscopy technology samples several thousand optical channels
simultaneously through a coded aperture instead of through a single-channel slit
(Figure 2). Then mathematical algorithms precisely reconstruct the intensity-versus-wavelength
information. Using this technology, both resolution and throughput can be maintained
and optimized. The most dramatic performance advantage is realized when measuring
weak, scattered or diffuse sources.
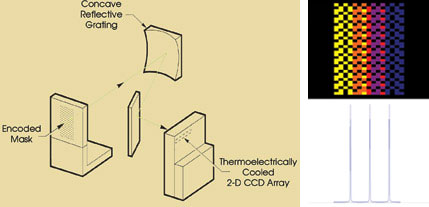
Figure 2. This schematic shows the optical layout of a multimodal,
multiplexed spectrometer (left), along with a typical CCD detector image and spectrum
(right).
In particular, for a Raman spectroscopy
system, theory predicts the throughput of an encoded mask to be about 800 times
that of a fiber-based system and 12 times that of a slit system with equivalent
resolution.
To validate this theory, data were
collected using a spectrometer comprising identical optical and electronic components,
with the only difference between experiments being the change in the entrance aperture.
The spectra of toluene — comparing pinhole, slit and multimodal multiplex
spectroscopy approaches — are shown in Figure 3. The multimodal, multiplex
spectroscopy technique provides high sensitivity and resolution.
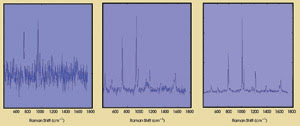
Figure 3. The Raman spectra of toluene with
pinhole (left), slit (middle) and multimodal, multiplexed (right) configurations
show how the latter technique improves the sensitivity and reproducibility of the
data.
Lateral flow immunoassay
Influenza virus causes significant illness and
mortality worldwide. According to the Centers for Disease Control and Prevention
in Atlanta, influenza infections result in more than 114,000 hospitalizations and
36,000 deaths annually and, together with pneumonia, represent the sixth-leading
cause of death in the US. A rapid and accurate means of detecting the virus is,
therefore, very important.
Current commercial methods for detecting
influenza include tissue culture isolation, which requires two to 14 days to complete,
or ELISA measurements, which require two to six hours. There also are various rapid
but less accurate lateral flow immunoassay tests.
In a traditional lateral flow immunoassay,
the sample to be analyzed is deposited on one end of a cellulose strip and the results
are read at the other. As the sample flows through the strip, an antibody containing
a spectroscopic tag typically attaches to the biomarker molecule. As the labeled
biomarker moves farther down the strip, it reaches a zone where it becomes attached
to another receptor that is fixed in place at the test/read point on the strip.
The test is then analyzed to determine the quantity of bound biomarkers by quantifying
the amount of spectroscopic tag that is present. Common measurement techniques include
colorimetry, chemiluminescence and fluorescence.
It also is possible to measure the
concentration of the biomarker using Raman spectroscopy, which, although traditionally
not a trace analysis technique, is very sensitive to low concentrations when combined
with surface-enhanced Raman spectroscopy.
Surface-enhanced Raman effects occur
when molecules are in proximity to certain metallic surfaces. The metal causes orders-of-magnitude
enhancement in the Raman signal. In an experiment to test the ability of Raman spectroscopy
to improve lateral flow immunoassays, the surface-enhanced particles were provided
by Oxonica plc of Yarnton, UK. The particles, which consist of a gold nanoparticle
core that is coated with the Raman probe molecule (that is, the reporter), are further
encased in a glass layer that stabilizes the Raman probe molecules.
In the Raman-based lateral flow immunoassay,
the antigen bound to the surface of the glass layer and the Raman signal was recorded
from the reporter molecule when the biomarker/antigen was bound at the measurement
point of the test strip (Figure 4).
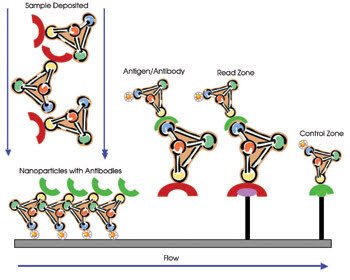
Figure 4. This
schematic shows the steps involved in a typical lateral flow assay, including sample
deposition, tagging and detection.
The test solution that includes the
biomarker was applied to the end of the test strip where it then bound to the labeled
nanoparticles. The labeled biomarker and the unattached tags flowed down the strip.
At the test point, the tagged biomarkers bound to receptors fixed on the strip,
and the reporter molecules were read. The unbound reporter molecules (the background)
were read at the control point. The difference between the two signals was used
to calculate the concentration of the biomarker and, therefore, the presence or
absence of an infection.
Another advantage of using the multimodal
multiplex method with Raman spectroscopy for bioassay measurements versus conventional
detection approaches is the spectral contrast that enables the measurement of multiple
Raman reporter molecules — hence, multiple biomarkers — simultaneously.
This is possible because of the narrow features of the Raman emission spectra.
Besides providing higher sensitivity
through increased spectral contrast, the multimodal, multiplex, wide-area-aperture
approach also is suited to large-area sampling. Typical Raman spectrometers use
an illumination spot size of ~100 to 150 μm. Many samples are not homogeneous
over such a small area and, as a result, the measured spectrum may not be representative
of the bulk of the sample.
For example, a surface-enhanced Raman
spectroscopy substrate was measured with a conventional Raman spectrometer that
had a spot size of ~100 μm and with a multimodal, multiplex system with
a sample spot size of 2 mm.
A series of spectra was collected across
the sample, using 500-μm steps (Figure 5). Using conventional Raman spectroscopy,
the spectral intensities show a wide variation because of the surface variations.
On the other hand, the much larger illumination spot size of the multimodal, multiplex
system resulted in more reproducible spectra.
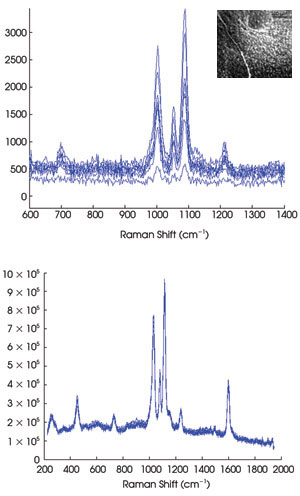
Figure 5. In a spectrographic analysis of
a sample surface covered with nanoparticles (inset), the Raman spectra acquired
using a typical slit-based system (top) offer less sensitivity than those acquired
using the multimodal, multiplexed system (bottom).
These results also demonstrate the
improvement in signal-to-noise that results from using the multimodal, multiplex
approach. Such uniform detection is a critical part of obtaining accurate, reproducible
bioassay measurements.
Using three Raman reporter nanoparticles,
it is possible to measure more than one virus at a time. For example, influenza
A, influenza B and respiratory syncytial virus are detectable in a single 15-minute
assay with a detection limit of approximately 10 ng/ml — an order of magnitude
better than current lateral flow immunoassays using colorimetric detection.
In summary, Raman spectrometry enhanced
with multimodal, multiplex technology provides approximately 12 times the throughput
of a conventional slit-based system with equivalent resolution. This translates
into a signal-to-noise advantage of more than 3 1/2 times for equivalent measurement periods. In addition, the wide-area aperture is suited to large sample spot illumination, which yields measurements that are more
representative of the bulk of the sample being analyzed. This greatly enhanced sensitivity
and larger area sampling make the multimodal, multiplex Raman spectrometry system
ideal for surface-enhanced bioassay measurements.
Acknowledgments
The authors thank Scott Norton and William Doering
at Oxonica for providing the samples used in these experiments and for their technical
help.
Meet the authors
Michael Fuller (e-mail: [email protected]) is
senior director of product development, Prasant Potuluri (e-mail: [email protected])
is a cofounder and chief technology officer, and Michael Sullivan (e-mail: [email protected])
is a cofounder and vice-president of business development of Centice Corp. in Morrisville,
N.C.