
‘Electrode Barrier’ Broken for Organic Solar Cells
AMHERST, Mass., Sept. 19, 2014 — A new organic solar cell has broken the “electrode barrier” known to hamper efforts to enhance efficiency.
A team from the University of Massachusetts Amherst developed the cell, which can use virtually any metal as the electrode. Many common metal electrodes can obstruct the power conversion efficiency of organic solar cells, as they are typically unstable and susceptible to oxidation.
“The sun produces 7,000 times more energy per day than we can use, but we can't harness it well,” said Dr. Thomas Russell, a professor of polymer science and engineering at UMass. “One reason is the trade-off between oxidative stability and the work function of the metal cathode.”
More stable metals that don’t degrade in the presence of water and oxygen have high work function, but do not allow good electron transport. Metals with lower work function and easier electron transport have been found to be unstable and over time will degrade and become less conductive.
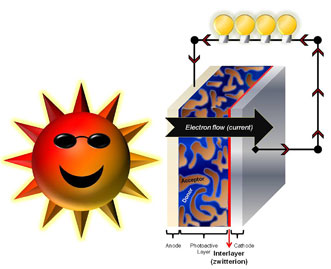
A layer of zwitterionic fullerenes is applied to allow the use of any type of metal electrode in a more efficient, lightweight and low-cost organic solar cell. Courtesy of the University of Massachusetts Amherst.
In their study, the researchers used ultraviolet photoelectron spectroscopy (UPS) to categorize several metals including copper, silver and gold, and to identify exactly what aids electron transport from solar cells’ photoactive layer to the electrode.
The researchers synthesized conjugated polymers featuring zwitterions — neutral molecules with both a positive and negative charge that also have strong dipoles that interact strongly with metal electrodes. These conjugated polymer zwitterions (CPZ) were applied to several different polymer scaffolds in conjugated systems in the inter-layer of solar cells.
“Once we could make CPZs, we were able to incorporate any conjugated backbone we wanted with zwitterionic functionality,” said Dr. Todd Emrick, a synthetic chemist and polymer science professor at UMass.
The researchers next turned to fullerenes, which are often used in the photoactive layer of solar cells and provide strong electron transport, to determine electron transfer efficiency. By modifying the fullerenes with zwitterions to change the work function of the electrodes, they said they were able to incorporate the zwitterions’ functionality as efficiently as possible.
“This is really a sweeping change in our ability to move electrons across dissimilar materials,” Emrick said, adding that the researchers essentially made “polymers and fullerenes that change the qualities of the metals they contact, that change their electronic properties, which in turn transforms them from inefficient to more efficient devices than had been made before.”
The research was published in Science (doi: 10.1126/science.1255826).
For more information, visit www.umass.edu.
Published: September 2014