The key to rolling back climate change could be the sun, according to George Washington University
researchers who have demonstrated a carbon capture process that promises to use
solar power to remove carbon dioxide from the air. As a bonus, the technique could
generate a profitable product.
“Because the process simultaneously uses both solar thermal
and solar visible, it captures and uses more solar energy than solar cells, and
it removes and converts carbon dioxide into a useful form,” said Stuart Licht,
a chemistry professor at the university’s Columbian College of Arts and Sciences
in Ashburn, Va.
Licht was lead author on a Journal of Physical Chemistry Letters
paper published online on Aug. 18, 2009, describing the process, which the researchers
dubbed solar thermal electrochemical photo, or STEP, carbon capture. It has a carbon
dioxide capture efficiency of up to 50 percent; i.e., as much as half of the energy
in sunlight can be used to remove CO2 from the air.
The end product is solid carbon or carbon monoxide. The latter
could be used as feedstock for synthetic jet, kerosene and diesel fuels as well
as for plastics and medicine. The revenue derived from selling such feedstock or
the solid carbon could change the economics of combating rising CO2 levels, making
mitigation much more attractive.
In a demonstration of the new carbon capture technique, the investigators
bubbled CO2 through an electrolysis cell filled with molten lithium carbonate. They
used concentrated solar thermal, or infrared, energy to heat the cell to a temperature
above the 723 °C melting point of the material, forcing the temperature as
high as 950 °C. They used photovoltaics to convert the visible part of sunlight
into electricity that drove the electrochemical reaction.
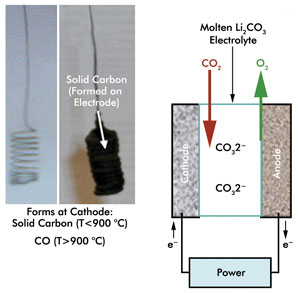
Sunshine powers an electrolysis cell (right) that turns atmospheric CO2 into solid carbon (left) or carbon monoxide. Courtesy of Stuart Licht, George Washington University. Reprinted from the Journal
of Physical Chemistry Letters.
The high temperature decreased the energy required for that reaction.
What’s more, the output of that reaction depended upon the temperature.
In their demonstration, the researchers plated out solid carbon
on the cathode of the electrolysis cell at temperatures as low as 750 °C. At
higher temperatures, the amount of solid carbon decreased and the production of
gaseous carbon monoxide increased. At 950 °C, only the gas was produced.
Because the technique is highly immune to poisoning effects, it
could be used to directly treat the waste stream coming out of a smokestack, something
that Licht noted was not possible with other proposed carbon capture methods.
Calculations showed that it is feasible with STEP carbon capture
to reduce CO2 in the atmosphere to preindustrial levels within 10 years. There is
enough annual lithium carbonate production to do this, although it would require
a significant fraction of the world’s output. Roughly 700 km2 of photovoltaics
would be needed. How long it would take to remove the CO2, Licht said, depends on
the commitment to capturing carbon.
The technique also could use potassium carbonate, of which there
is a much larger potential supply than there is of lithium carbonate. The downside
is that using potassium would require higher electrolysis potentials and would therefore
be not as attractive as the lithium alternative.
The STEP method also could reduce atmospheric CO2 in other ways.
Licht and his team showed in a Chemical Communications paper published online on
Aug. 23, 2010, that a variation of the STEP technique can produce pure iron from
two common ores, hematite and magnetite, without emitting carbon dioxide. This approach
could significantly reduce the estimated 6.8 billion tons of CO2 emitted each year
by the iron industry.
Licht said that the group welcomes commercial or governmental
inquiries about the STEP technique. He added that the method can be used for environmentally
benign generation of fuels, a process that requires teaming it up with solar-energy-produced
hydrogen.
Noting that the extraction of CO2 from the air is the culmination
of efforts stretching over 20 years, he said, “Until now, it has been a challenge
to convert the stable molecule carbon dioxide into a useful product and remove it
from the atmosphere. It is exciting to watch carbon dioxide be bubbled into the
STEP process and be easily converted into solid carbon.