Full company details
Excelitas Technologies Corp.
 Corporate Headquarters
Corporate Headquarters
2545 Railroad St., Ste. 300
Pittsburgh, PA 15222
United States
The Evolution of Fluorescence Illumination Is Refining Medical and Research Applications
BioPhotonics
Mar/Apr 2025The excitation of fluorophores, combined with white light illumination, has advanced analytical instrumentation, medical diagnostics, endoscopy, and surgical procedures.Kavita K. Aswani, Excelitas Technologies Corp.
Innovations in biomedical illumination technology are transforming various aspects of medical procedures, imaging techniques, and therapeutic interventions. Improved illumination enables surgeons to perform complex procedures with greater precision and better patient outcomes, particularly in minimally invasive and robot-assisted procedures. In this context, advanced techniques in fluorescence allow many advantages, exciting the fluorophores within biological samples through external dyes, genetically modified fluorescent molecules, or leveraging intrinsic autofluorescence. When fluorescence imaging is applied in a research or health care setting, it offers exceptional specificity, providing detailed molecular-level information that surpasses the data produced by traditional bright-field hematoxylin and eosin sample staining techniques.

Surgeons use every tool at their disposal, including various illumination systems. Courtesy of iStock.com/gorodenkoff.
Past limitations
For several decades, xenon and halogen bulbs were the gold standard in illumination for many medical procedures. Xenon bulbs provided the brightest illumination and “closest to sunlight” color rendering for biomedical applications; however, their limited lifetimes and thermal risks were drawbacks that made room for LED and laser technologies in this application space.
LEDs offer a more reliable and stable light source
with a longer lifetime and fewer consumables than
the incumbent technology.
In their infancy, solid-state LED illumination sources were not viable replacements for xenon and halogen bulbs. The gaps in costs, output power, and quality of white light were limitations that improved over time. The green gap played a huge part in the slow adoption of this technology in the life sciences: LEDs had very poor optical output within the 500- to 600-nm region. Over time, brighter LED options and laser phosphor conversion technology helped to increase output at these wavelengths, which are important in biomedical applications. LEDs now offer a more reliable and stable light source with longer lifetime and fewer consumables than the incumbent technology
1.
Advantages of LED use
The shift from traditional lamps to LED technology is influenced by several key factors and provides the following advantages.
• Longevity and durability
LEDs have a longer lifespan than traditional lamps. While xenon and mercury lamps typically last between 200 to 2000 h, LEDs boast instant on/off capabilities with no warm-up time, allowing operation for tens of thousands of hours, reducing the need for frequent replacements and maintenance.
• Wavelength specificity
LEDs can produce precise wavelengths to create a specific white from red, green, and blue LEDs. They can also be tailored to match the exact fluorescence wavelength needed for excitation. This is useful in medical diagnostics in which specific wavelengths are required for enhanced tissue visualization and contrast. The tunability of LEDs and the ability to combine the light from separate LEDs allows for precise color rendering and customization of correlated color temperature, improving the accuracy of medical imaging.

An illustration of various color-corrected temperatures. Different color temperatures provide different shades of white. Household bulbs, for example, can be warm white or cool white. Courtesy of iStock.com/Veronika Oliinyk.
• Reduced heat generation
Endoscopic cameras or overhead cameras are used to
detect fluorescence and provide real-time visualization during a live surgery.
LEDs produce less heat than traditional lamps, but the heat generated by LEDs must be carefully managed, because their emission characteristics are more sensitive to temperature. Heat generation and management is important in medical or analytical applications in which excessive heat can have an effect on other equipment in a surgical suite, or within an endoscope, to the detriment of the image being produced.
• Compact size and integration
LEDs are smaller than lamps and are becoming available in a great variety of compact assemblies every year. This allows for smaller equipment and more of these units to be used in diagnostic facilities, making diagnosis increasingly efficient. In surgery, smaller endoscopes equate to increased patient comfort and faster recovery times.
• Energy efficiency
LEDs are more energy-efficient compared with traditional halogen, metal halide, or xenon lamps. They consume less electrical power while providing the same or higher levels of illumination output, reducing operational costs and the environmental footprint.
Fluorescence in biomedicine
Fluorescence in biomedical and life sciences has evolved significantly since the development of the first synthetic fluorophore in 1871
2. Histology, including antibodies (immunohistochemistry), provides specificity, but not the signal sensitivity of fluorescence, which revolutionized biological research by enabling the precise location of proteins in cells. Autofluorescence in the UV/blue region necessitated the creation of fluorophores that are more identifiable in the green or red emission range of the spectrum. Protein labeling gave way to labeling chromosomes and then DNA using fluorescence (fluorescence in situ hybridization), providing detailed specificity and visualization of genetic material that had never been seen.
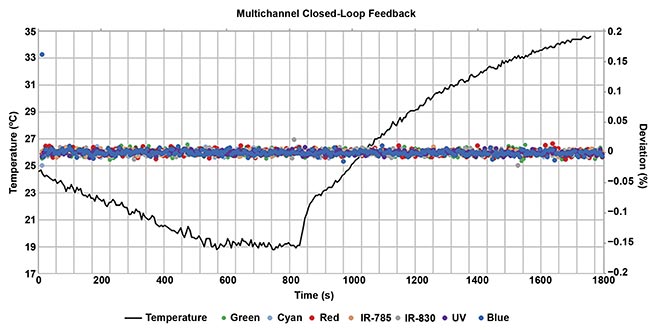
An example of an illuminator with and without closed-loop feedback. The black line represents temperature changes. The dots represent deviation of optical output with closed-loop feedback ‘on.’ As temperature changes, the optical output or power of the LED remains the same. Courtesy of Excelitas.
The discovery of the green fluorescence protein in 1961 by Osamu Shimomura further transformed the research world. This fluorophore allowed scientists to manufacture proteins in a cell with fluorescence attached to them, without having to damage the cell with the introduction of an external fluorescence molecule. This was the birth of the green fluorescence protein and all the fluorescence proteins that have been engineered since.
Advancements in available wavelengths and types
of illumination will continue, enabling imaging deeper
into tissues with more specificity and less damage to
the tissues.
What began as a more sensitive marker for antibodies has transitioned to the medical and diagnostic fields, offering enhanced specificity and sensitivity for medical diagnoses.
Analysis and medical diagnostics
Fluorescence is widely used in analytical and medical diagnostic instrumentation due to its high sensitivity and specificity. Applications of fluorescence illumination in the analytical/diagnostic arena include, but are not limited to, fluorescence microscopy/detection for molecular or cellular imaging — screening hundreds of samples, e.g., COVID tests, fluorescence in situ hybridization (FISH), dynamic studies; cell sorting and analysis, e.g., isolating stem cells; in vivo diagnostic imaging (this can be based on fluorescence, but also can be label-free, relying on intrinsic fluorescence of biological compounds); food and drug analysis, e.g., quality, pharmacokinetics, toxicology; and endoscopy and surgery.
Surgical visualization
Fluorescence-guided surgery is a medical imaging technique used to help surgeons visualize and distinguish vasculature or tumor tissue from normal tissue during surgical procedures. It allows the surgeon to clearly see tumor boundaries, helping to achieve more complete tumor removal while sparing healthy tissue and vasculature.
The technique involves administering a targeted fluorophore to the patient before or during surgery. Endoscopic cameras or overhead cameras are used to detect fluorescence and provide real-time visualization during live surgery.
Limitations of fluorescence in this context can include restricted tissue penetration depth and availability of tumor-specific fluorescent agents. It is an active area of research, with new fluorescent probes and imaging systems under development to expand its capabilities and applications
3.
The following fluorophores are now commonly applied to fluorescence-guided surgery:
Indocyanine green (ICG): ICG has been FDA-approved since 1956 and used in surgery and ophthalmology to identify blood and lymphatic flow. Use of NIR wavelengths to excite this fluorophore allows for deeper tissue penetration and higher signal-to-noise ratio in resulting images. ICG is used to clearly mark vasculature to ensure less damage to blood vessels during surgery; it can also indicate ischemia or stroke (lack of blood flow), or the presence of a tumor (increased blood flow).
Protoporphyrin IX (PpIX) and 5-aminolevulinic acid (5-ALA): When 5-ALA is introduced into the body, it is metabolized into PpIX and accumulates in cancer cells. When excited with blue light, PpIX emits red fluorescence, thereby helping the surgeon differentiate tumor tissue from normal tissue for thorough tumor resection.
Although less specific, fluorescein and methylene blue are used to visualize structures during surgery. Methylene blue is used to view lymph nodes for tracing lymphatic flow and drainage, parathyroid glands, ureters, fistulas and sinuses, as well as general tissue blood perfusion.
Fluorescein tends to accumulate in areas with a compromised blood-brain barrier and is therefore useful for identification of gliomas or other brain tumors where the barrier is disrupted. Fluorescein and methylene blue simply accumulate due to changes in physiological characteristics such as increased vascularity or disrupted barriers. They are primarily used to enhance overall visualization of structures during surgery rather than targeting specific tissues with high selectivity.
Light source requirements
The specific requirements for each light source will depend on the intended application, ranging from simple single-wavelength devices to complex, multichannel illuminators capable of advanced measurements. Regulatory requirements for the final product, and the parameters to be stored for recordkeeping and liability, also play a factor.
Light source requirements can be determined by assessing the following:
What is being imaged? A white light or combined RGB source is required for basic bright-field images of structures that are optically unclear. Fluorescence would require specific wavelengths of LEDs or lasers to excite the fluorophore of interest, and there may be filters involved to further narrow down the LED spectrum. Combining the two allows for observation of the anatomy with fluorescence of interest overlaid on the bright-field image.
Anatomy of the light source: What is the number of wavelengths required simultaneously or sequentially? If imaging sequentially, the light source must be able to turn each LED on and off, or turn one or more on at a time. The numerical aperture (angle) of the light emitting from the light source and into the optical path of the instrument will dictate efficiency of the light entering the optical train.
Footprint: Where is the light source residing? Size is an important factor if the light source is within a larger scanning system or in an operating suite rack.
What color is white? Color-corrected temperature indicates the color of light emitted by a light source. It indicates the warmth or coolness of the light, which is most important in surgical applications for the best tissue contrast. The color rendering index indicates how close the light source is to natural light. This is crucial for the surgeon to see subtle differences in the natural color of organs and tissue, and for identifying red tones indicative of blood and vital organs.
Control: Speed of switching, control through external triggering and sources of control, and automation capabilities for high-throughput experiments are important considerations. High power equates to reduced exposure times, enabling fast switching between colors and detection of more samples in a short duration of time.
Stability and uniformity: Light source stability and uniformity encompasses output stability and wavelength (drift) stability, both of which rely on thermal stability of the illumination system. A thermally stable system will control the effects of heat generated on wavelength, optical stability, and the lifetime of the LEDs and lasers.
Feedback: While degradation of LEDs is minimal, output power can and does gradually change over time. Incorporating closed-loop feedback in a light source ensures the output can be maintained and will be adjusted automatically in a system. This is crucial in diagnostic and surgical illumination, where optical output fluctuation could affect resulting tests or produce an adverse effect on a live patient.
While light source design varies by application, the system components along with the instrument as a whole must be considered. An illuminator can range from being a simple LED source with fiber delivery to complex multiwavelength systems for diagnostic or surgical applications. For example, a light source residing within a larger diagnostic scanning instrument would be a more complex system, with advanced thermal management of the LEDs. Control of this light source (spectrum, wavelength switching speed, power output) through external sources would be critical for its operation and to increase scanning throughput.
As mentioned above, attaining feedback control to maintain the optical power of light reaching each sample is crucial in diagnostics. Adaptability to the cloud, Ethernet, Internet of Things, or artificial intelligence should also be considered when designing new illumination systems. These tools can help with real-time collaborative diagnosis and automatic image recognition — tools to help the surgeon create the optimal outcome for the patient. The possibilities are limitless, and the industry will continue to adapt to meet these complex needs while continuing to grow.
Future integration
Advancements in fluorescence imaging have expanded its applications beyond traditional microscopy, revolutionizing medical diagnostics and treatment. Advancements in available wavelengths and types of illumination will continue, enabling imaging deeper into tissues with more specificity and less damage to the tissues. In an ideal world, external fluorophores would not need to be introduced into a sample or patient. Ongoing research shows that using dark-field microscopy or advanced phase contrast techniques can allow for label-free imaging, which reduces external factors to enable more accurate studies of cells, tissue, or organs in their natural state. As we look to the future, the field is destined for further innovations. Researchers are developing techniques to image deeper into tissues with greater specificity and minimal damage.
Lastly, the integration of artificial intelligence (AI) with these imaging techniques is enhancing image processing and analysis, opening new realms for automated diagnostics. AI allows for real-time surgical guidance and collaboration between specialists. As these technologies continue to evolve, they promise to provide new insights into biological processes, disease mechanisms, and treatment efficacy, ultimately leading to improved patient outcomes.
Meet the author
Kavita Aswani is the senior biomedical applications scientist for biomedical products at Excelitas Technologies. She earned her doctorate in anatomy and cell biology from the University of Iowa and has more than 25 years of applications experience in the field of microscopy and in the fluorescence industry; email:
[email protected].
References
1. K. Aswani. (2023). Illumination Advancing Fluorescence Microscopy in Life Sciences,
Medical Realms.
BioPhotonics, Vol. 30, No. 5, pp. 28-33.
2. R.Y. Tsien. (1998). The Green Fluorescent Protein.
Annu Rev Biochem, Vol. 67, pp. 509-544.
3. C. Ewelt et al. (2015). Fluorescence in neurosurgery: Its diagnostic and therapeutic use. Review of the literature.
J Photochem Photobiol B, Vol. 148, pp. 302-309.