Full company details
Edmund Optics
 101 E Gloucester Pike
101 E Gloucester Pike
Barrington, NJ 08007
United States
Phone: +1 856-547-3488
Fax: +1 856-573-6295
Toll-free: +1 800-363-1992
Raman Spectroscopy Identifies Disease Characteristics and In Vitro Structure
BioPhotonics
Mar/Apr 2023A successful setup depends heavily on selecting the correct laser wavelength as well as the proper mirrors, lenses, prisms, and other components to examine a sample’s composition.Emily Bishop, Edmund Optics
Since the invention of lasers and their integration into Raman spectroscopy systems, the spectroscopy technique has grown in popularity for use in life science and medical applications as a way to noninvasively analyze a sample and identify the component parts. The Raman effect is best measured with monochromatic light sources such as lasers because the wavelength of the scattered photons is different than that of the exciting laser source. This means that the wavelength of the light source is a key specification to consider when developing a Raman spectroscopy system.

Raman spectroscopy is used in many biomedical
applications, such as when analyzing the biochemical environment of cells to see how cells react to
pharmaceuticals. Courtesy of Edmund Optics.
A high signal-to-noise ratio (SNR) is beneficial for detecting the low efficiency of Raman shifts, which are frequency shifts that occur when monochromatic light scatters off a sample and produces a different frequency than that of the original light source. A high SNR is especially important for the analysis of fluorescent samples, which are notoriously difficult to analyze using Raman spectroscopy because fluorescent material produces a much more intense signal than the weak Raman signal. This causes the Raman signal to be overpowered and the data collected to be less useful.
To aid in extracting the Raman signal, a laser with a wavelength that is longer than that of the energy gap between the electronic ground and excited states should be chosen. The detector’s sensitivity should also be considered. For example, when using a 532-nm laser, the resulting scattered photons will be distributed in the visible range, and, thus, a detector should be chosen that has high quantum efficiency in the visible spectrum, such as a charge-coupled device (CCD). However, when using a NIR laser such as an Nd:YAG with a wavelength of 1064 nm, indium gallium arsenide (InGaAs) detectors are ideal due to their high sensitivity in the NIR spectrum.
Virtually every Raman setup includes a laser to excite the sample and a detector to collect the emission signal. Additional optics are integrated into the system to focus the beam and optimize the signal. A simple Raman spectroscopy setup may include an Nd:YAG laser, two mirrors on kinematic mounts, two right-angle prisms, and an achromat lens. The light from the Nd:YAG laser hits two mirrors, as shown in the configuration illustrated in Figure 1, and is reflected 90° by a right-angle prism. An achromatic lens then focuses the light onto the sample. The light that scatters off the sample hits the second prism mirror, which deflects it into a beam dump. The achromat then gathers the scattered light and focuses it onto the detector for collection
1.

Figure 1. A common layout for a Raman spectroscopy system. Adapted with permission from Reference 1.
The power of NIR lasers can get up to the hundreds of milliwatts, so it is critical to make sure the laser mirrors, lenses, and filters used in the Raman spectroscopy system have a high enough laser damage threshold to be compatible with the particular laser source being used. However, it is important to realize that, because of the statistical nature of laser damage testing, this threshold is not the power or fluence below which damage will never occur, but rather the limit at which the damage probability is less than a critical risk level. The level of risk depends on several factors — such as the beam diameter, the number of test sites per sample, and the number of samples tested — to determine the specification.
In Raman spectroscopy, a clean excitation signal is a vital component in an experiment to ensure that scatter data is measured accurately. To ensure that only the desired signal is detected, high-performance bandpass and longpass filters complement each other very well in this regard when incorporated into a system (Figure 2). The high transmission and narrow bandwidth of the bandpass filters eliminate signal noise and ensure that only the desired laser line reaches the sample. The high blocking and narrow transition of laser line filters then eliminate the excitation signal and allow accurate measurement of wavelengths very close to the laser line.
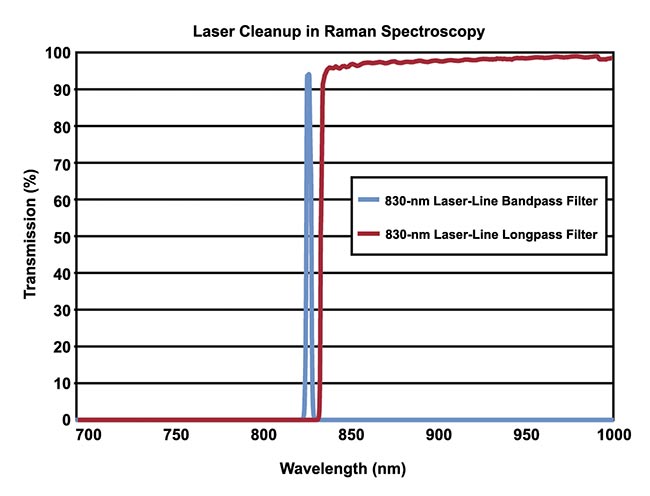
Figure 2. The bandpass filter is used to pass the excitation wavelength, and the longpass filter is used to block the excitation wavelength later on in the system while allowing longer scattered wavelengths to pass. Courtesy of Edmund Optics.
Key filter specifications to look for are the transmission inside the desired waveband and the optical density (OD) of the blocking range. A high OD value indicates low transmission, and low OD indicates high transmission. ODs of six or greater are typically needed for the high blocking required in Raman spectroscopy. The percentage of light transmitted is given by 10
−OD × 100%, so OD can be calculated by using the following formula:
 Basic principles of Raman
Basic principles of Raman
The Raman spectroscopy technique was developed to identify the chemical composition of a sample based on the light-matter interaction process of Raman scattering. Raman scattering is a physical process in which the direction and energy of incoming light changes as it hits a sample. Unlike Raleigh scattering, Raman scattering is an inelastic form of scattering, meaning that kinetic energy from the incident light is not conserved.
In Raman spectroscopy, the scattering effect is used to analyze a substance and determine its structural composition. The technique is commonly used in the life sciences and medicine to analyze single cells and drug-cell interactions, and in solid-state physics and chemistry to identify both organic and inorganic materials. A key benefit of Raman spectroscopy is that it is a noninvasive, nondestructive way to assess samples without using dyes or labels. Additionally, it can be used with any sample composition, so no sample preparation is needed.
When incoming light hits a sample, most of the light is absorbed or transmitted through the sample. However, a small amount of light is scattered in all directions, and it can be captured by a sensor. The scattered light follows one of three patterns: Rayleigh, Stokes Raman, or anti-Stokes Raman (Figure 3). When the scattered frequency is equal to that of the incident frequency, this is known as elastic scattering. Rayleigh scattering is a type of elastic scattering. Inelastic scattering occurs, however, when energy is not conserved.
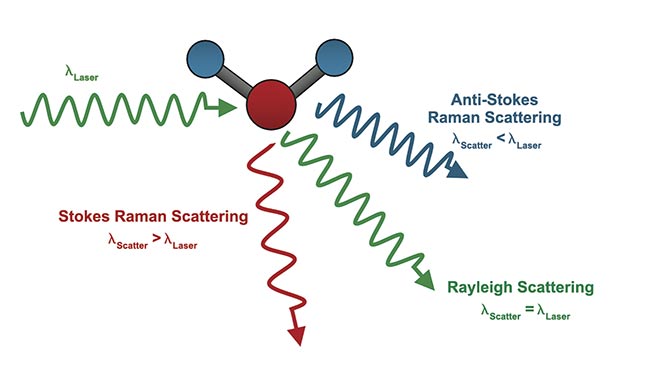
Figure 3. A comparison of Rayleigh, Stokes Raman, and anti-Raman Scattering, where λlaser is the wavelength of the laser source and λscatter is the scattered wavelength. Courtesy of Edmund Optics.
When the molecule gains energy from the scattering of photons, the molecule is excited to a higher vibrational state. This effect is known as Stokes Raman scattering. When the molecule loses energy, the effect is known as anti-Stokes Raman scattering. Stokes Raman scattering tends to have a higher intensity than anti-Stokes Raman scattering, and, thus, Stokes scattering is typically used in Raman spectroscopy measurements. In this spectroscopy technique, a sample is excited by a monochromatic light source, such as a laser, and the change in vibrational frequency is measured to create a unique fingerprint from which the sample can be identified.
Raman vs. FTIR spectroscopy
Raman spectroscopy is often confused with Fourier-transform infrared (FTIR) spectroscopy by practitioners in both life science research and industrial applications but who do not specialize in Raman. FTIR spectroscopy is one of the most commonly used techniques for infrared spectral analysis, such as when determining the ingredients of compounds or identifying contaminants. FTIR spectroscopy is also based on changes in vibrational energy. However, the mechanism by which the signal is produced is different than in Stokes Raman spectroscopy.
In Stokes Raman, photons are absorbed when light interacts with a sample, causing the molecule to rise to a higher energy virtual state. At this state, lower-energy photons are emitted, causing the molecule to shift to an excited vibrational state. The associated energy difference between absorbed and emitted photons corresponds to that between the ground and vibrational energy states. But in FTIR spectroscopy, light hits a sample and is absorbed without a virtual state change. The radiation that is absorbed by the molecule directly corresponds to the energy difference between two vibrational states and the energy of an allowed vibrational transition.
Another major difference between FTIR and Raman is in the type of samples that the technique is used to analyze. Raman spectroscopy is difficult to use with fluorescent samples because the fluorescent signal is much more intense than the Raman scattering. However, Raman spectroscopy is better suited for analyzing aqueous samples than FTIR spectroscopy because water produces very weak Raman signals, making it easier to identify signals from the areas of interest. Additionally, Raman spectroscopy requires little to no sample preparation, so it is ideal for new system users, while FTIR spectroscopy requires careful sample preparation.
Advanced Raman techniques
A commonly used variation of Raman spectroscopy for ultrasensitive sensing applications such as analysis of DNA, viruses, and other biomolecules is surface-enhanced Raman spectroscopy (SERS). This technique optimizes the Raman scattering of molecules adsorbed on a nanostructured metal surface (silver, gold, or copper). Signal enhancement is achieved by using two processes: the electromagnetic mechanism from the metal surfaces and chemical enhancement by charge-transfer mechanisms. The result is a highly optimized signal to the degree of approximately 10
10 to 10
11. Since normal Raman scattering produces a very weak signal, the technique amplifies the emitted signal for ease of detection.
In a combined system, Raman spectroscopy is also frequently integrated into microscopy techniques (such as confocal microscopy) using a laser-based Raman microscope. The microscopes used in these instances differ from traditional microscopes in that they include components such as an excitation laser, laser filters, and a spectrometer with some type of detector (usually a CCD or a photomultiplier tube). Using Raman spectroscopy at the microscopic level is beneficial for analyzing microscopic structures for chemical identification. The Raman setup can be integrated into the regions in which light is collimated after leaving an infinite conjugate objective and before reaching the tube lens. Longer tube lengths may be beneficial for fitting the additional components into the system.
Raman spectroscopy is also useful for enhancing traditional biomedical imaging. With the aid of the Raman technique, imaging can be performed noninvasively, in vitro, to allow for visualization of biological structures. Additionally, high-resolution images can be obtained using confocal microscopy in conjunction with Raman spectroscopy. This has led to improved spatial resolution for 3D imaging
2.
Growing applications
Because Raman spectroscopy can be used to analyze a vast array of materials, it may be used in a variety of applications, such as in the life sciences to diagnose diseases and to identify the composition of medicines. Raman spectroscopy is also used in other disciplines, such as in chemistry to identify structures at the molecular level, materials science to view the stress and strain of a material, geology to identify minerals, and art and archaeology to identify unknown pigments or materials. Portable, hand-held Raman spectroscopy devices are also helpful when used on-site to quickly identify specimens, such as in forensic analysis or in point-of-care clinical settings.
Because the spectrum obtained through Raman spectroscopy provides accurate, fingerprint-like identification of specific molecules, the technique is powerful for differentiating between different types of cells and observing proteins and lipids inside cells. Any sample composition can be used, without any preparation or added markers, which greatly improves the ease of use.
Raman spectroscopy is being increasingly used in clinical disease detection. Healthy tissue and cancer cells have noticeably different spectra, allowing clinicians to distinguish malignant breast, lung, skin, and digestive system tissue. In vivo measurements not only eliminate the need for biopsies and lab analysis, they also produce results more quickly than conventional techniques. This leads to increased efficiency and a better patient experience.
NIR Raman spectroscopy is used for noninvasive brain analysis by making measurements through thin regions of the skull. For example, the concentration of neurotransmitters in a given area can be measured with high precision. However, the weak signals resulting from Raman scattering are even weaker after passing through the skull. More advanced techniques can amplify these signals, such as surface-enhanced Raman spectroscopy, which uses nanostructured materials for signal enhancement.
Raman spectroscopy is a powerful technique for nondestructively identifying unknown substances. When assembling a Raman system, it is vital to select the right mirrors, lenses, and prisms to minimize noise and light loss and meet the application’s requirements. From archaeology to the diagnosis of disease, this laser-based diagnostic method is critical in a wide variety of industries.
Meet the author
Emily Bishop is a product support engineer at Edmund Optics. She guides customers through the process of finding the right optical components for their application. Bishop has a bachelor’s degree in photographic sciences from the Rochester Institute of Technology; email:
[email protected].
References
1. R. Bausinger (2015). Raman spectroscopy setup and experiments for the advanced undergraduate lab. Proc ETOP, Article No. TPE31.
2. S. Wachsmann-Hogiu et al. (2009). Chemical analysis in vivo and in vitro by Raman spectroscopy — from single cells to humans. Curr Opin Biotechnol, Vol. 20, No. 1, pp. 63-73,
www.doi.org/10.1016/j.copbio.2009.02.006.