Long known for its ability to effectively examine molecular structure, STED has been adapted to acquire spatial and temporal data, helping to unveil the mystery of intercellular communication.
Stimulation emission depletion (STED) microscopy is a mainstay in superresolution imaging, since scientists established its capability to drastically improve the lateral resolution present in traditional fluorescence microscopy. In recent years, it has evolved into a method that can also be adjusted to capture the temporal and spatial data that is essential for studying rapid molecular dynamics in real time. Scientists are using STED in modern research to observe the way in which DNA binds and how these basic, but intricate, building blocks interact with each other in their biological environment. Often leveraging deep learning algorithms, these observations can lead to a greater understanding of the sophisticated mechanisms by which life evolves and adapts to its surroundings.
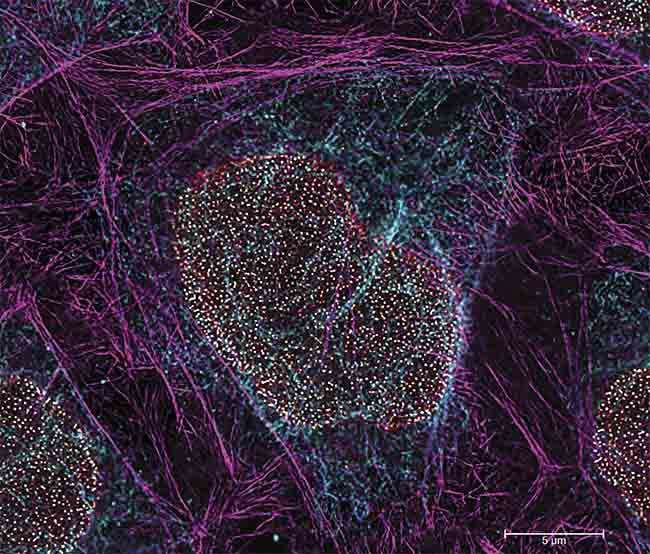
A multicolor image acquired using TauSTED Xtend, which includes Vimentin AF 594 (cyan), Phalloidin ATTO 647N (magenta), and NUP107 CF680R (glow). Triple color stimulation emission depletion (STED) imaging with a single depletion line at low STED power. Scale bar: 5 µm. Sample courtesy of Ludwig Maximilian University. Courtesy of Leica Microsystems.
STED microscopy is a superresolution technique in which a focused laser beam excites fluorescence in a small diffraction-limited region of a sample, and then a second red-shifted laser beam causes the surrounding fluorophores to return to their ground state, effectively switching them off. Given that the two beams overlap, a filter allows only the fluorescent photons from reaching the detector. This method creates a so-called doughnut hole of high resolution.
The technique was first described in a paper published by Stefan Hell and Jan Wichmann at the University of Turku in Finland in 19941. Hell, along with Eric Betzig and William Moerner, won the Nobel Prize in Chemistry in 2014 for helping to break the diffraction limit of 200 nm, which enabled a number of scientific discoveries regarding cellular structure and dynamics.
STED microscopy is a superresolution technique in which a focused laser beam excites fluorescence in a small diffraction-limited region of a sample, and then a second red-shifted laser beam causes surrounding fluorophores to return to their ground state, effectively switching them off.
Additional superresolution methods that were acknowledged at that time included structured illumination microscopy, a wide-field imaging technique in which a grid pattern is generated through interference of diffraction orders and superimposed on a specimen; stochastic optical reconstruction microscopy (STORM), a single-molecule localization technique in which a subset of labeled fluorescent molecules is activated by laser light while others remain dark; and photoactivated localization microscopy (PALM), a method related to STORM that identifies individual molecules through stochastic excitation via a lower-power laser beam and reconstructs an image over hundreds of frames.
Applications of STED have included the examination of the nodes of Ranvier, which are gaps in the myelin sheath that covers the axons of neuron cells. These gaps allow ions to diffuse in and out of neurons, facilitating signal recharge between neural cells. Disruption in the function of the nodes of Ranvier has been linked to the progression of neurodegenerative diseases. STED has also been used to study chromatin, which is made up of proteins and DNA and forms a complex structure within a cell’s nucleus. Chromatin helps facilitate the segregation of cells as mitosis occurs.
Time-resolved information
While the instrumentation of STED microscopy has been more complex than that of other superresolution methods, it has been standardized for use in many research laboratories around the world. STED systems include pulsed lasers, a single-photon detector (often, a single-photon avalanche photodiode, or SPAD), dichroic mirrors, a phase plate, an objective, a data acquisition unit, and a scanner (Figure 1). PicoQuant, among other companies, offers STED capabilities essentially as add-ons to their open and modular confocal microscope platform MicroTime 200, which is routinely used for time-resolved imaging in application areas ranging from materials science to life sciences (Figure 2).
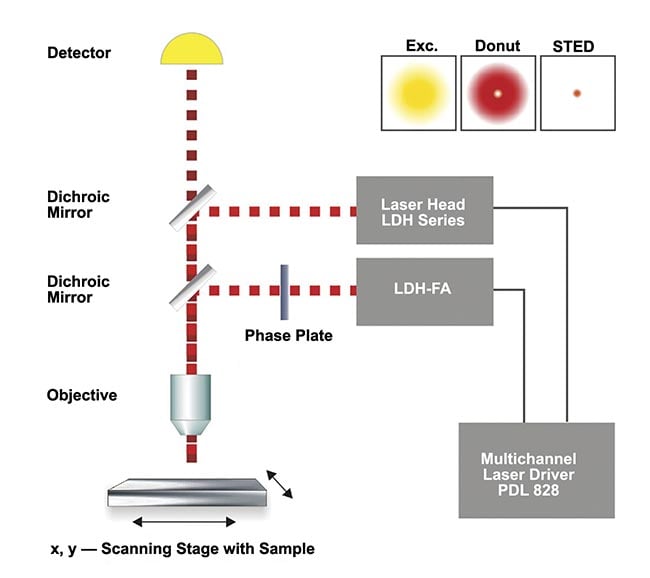
Figure 1. A schematic of a typical stimulation emission depletion (STED) setup. Exc.: excitation. Courtesy of PicoQuant.
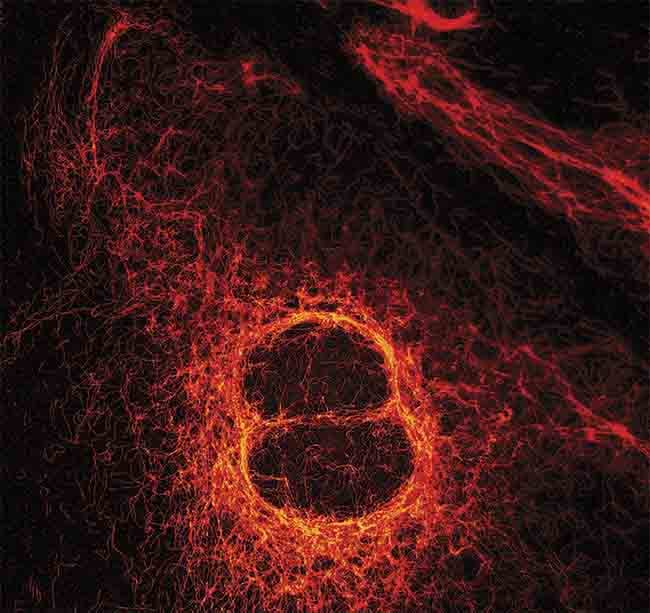
Figure 2. A stimulation emission depletion (STED) image of vimentin fibers, taken with the MicroTime 200. The fibers aid in the positioning and function of cellular organelles. Courtesy of PicoQuant.
Maria Loidolt-Krüger, a scientific content creator at PicoQuant, worked on her doctoral thesis in Hell’s group at the Max Planck Institute for Biophysical Chemistry in Gottingen, Germany. During this process, she helped develop multicolor STED microscopy and an associated live-cell staining protocol.
“A STED microscope is basically a confocal microscope with an additional depletion laser and a phase plate (or deformable mirror) to shape the STED beam into a doughnut focus,” she said. “At PicoQuant, we use the SPADs in single-photon counting mode to acquire time-resolved data.”
She said that the company’s microscope design is based on its working knowledge of single-molecule imaging and temporal resolution, and it incorporates time gating to eliminate photons spontaneously emitted before the STED beam is activated. She said that the MicroTime 200 can be used for fluorescence methods aside from imaging.
Researchers often apply STED in conjunction with other techniques, such as fluorescence lifetime imaging microscopy (FLIM) and fluorescence correlation spectroscopy (FCS) to examine protein-protein interactions.
“These different but compatible techniques yield different pieces of complementary information about the sample,” Loidolt-Krüger said. “STED increases the spatial resolution of the image to better localize protein-protein interactions. FLIM is often used for either multiplexing or discriminating spectrally similar fluorophores by their lifetime — they need to emit in the same range to be efficiently depleted by a single STED beam — or for monitoring local environmental changes via sensor fluorophores that change their lifetimes. FCS measures the concentration of a labeled, diffusing species or reports on its diffusion behavior (speed and mode).”
She said STED-FCS can be employed to monitor hindered diffusion at the cell membrane, where not all molecules pass through, based on factors such as size and temperature.
Giving STED space
Hell’s research team has continued expanding the utility of STED’s superresolution capabilities by adapting it for the integration of spatially resolved data. In the researchers’ recently published work, they described a technique called MINSTED, in which the co-aligned beams of a STED microscope (excitation and STED beams that form the point spread function) are adjusted in a circular motion around the fluorescent molecule. An FPGA was used to adjust for the number of detected fluorescent photons for each scanning position.
As STED microscopy has matured in scientific circles, many practitioners of the technique have increasingly turned to it for its capabilities in live-cell imaging, which can provide direct insight into cellular behavior, its evolution, and how cells could respond to potential therapeutics.
They found that by blue-shifting the STED beam and separating fluorophores with off-on switching, they could localize individual fluorophores attached to a DNA strand — traditional STED does not function with only a single fluorophore switched “on” at a given time. Given that they were able to achieve this single-digit nanometer resolution with a limited number of collected photons, researchers believe that this will enable MINSTED to be used to uncover a variety of information about subcellular structures2.
“MINSTED is a STED-based technique similar to MINFLUX [see sidebar], that uses detected fluorescence photons about 100 times more efficiently than widespread camera-based localization to identify the position of a fluorescent molecule,” Hell said. “It was the first technique to attain a localization precision less than 5 Å, with less than 5000 detected photons. That is simply not possible with popular camera-based localization techniques.”
He said that the Heisenberg uncertainty principle, conceived by the physicist Werner Heisenberg, states that the position and movement of an object cannot be precisely measured at the same time. Therefore, many photons are needed to demonstrate the position of a fluorescent molecule.
“In MINSTED, like MINFLUX, a large part of the burden of requiring many photons is taken away from the fluorescent molecules and put on the laser, which has no limits in providing photons,” Hell said.
This method could be used to track specific cellular processes at 1-nm resolution, and at a rate far faster than what has historically been possible.
Live-cell imaging
As STED microscopy has matured in scientific circles, many practitioners of the technique have increasingly turned to it for its capabilities in live-cell imaging, which can provide direct insight into cellular behavior, its evolution, and how cells could respond to potential therapeutics. Leica Microsystems, for example, has created a platform that capitalizes on multiple colors for this purpose.
The Leica system, called TauSTED, collects spatial and lifetime information to examine live specimens at the nanoscale level in conjunction with its STELLARIS 8 FALCON FLIM Microscope. This simultaneous assembly of data allows the technique to work with limited light, granting more time to view biological processes in a live specimen, with the use of green fluorescent proteins and other fluorophores that are standard in life sciences research (opening image).
“TauSTED Xtend ensures that live-cell experiments can be carried out over a longer time span thanks to the balance of light exposure and resolution, which leads to gentler imaging at stunning nanoscale depths,” said James O’Brien, vice president of life sciences and applied solutions at Leica.
An application report produced by Leica explained that the TauSTED method is based on the way STED affects the point spread function, and the way light is distributed from the center of the STED excitation beam. This readout provides valuable lifetime information without having to increase the intensity of the beam. TauSTED Xtend pairs the point spread function with an algorithm to unblur the image.
“This offers new opportunities for single- and multicolor experiments with green fluorescence proteins and fluorophores that are a workhorse in life sciences research and currently underused in nanoscope studies,” said Ulf Schwarz, application manager confocal microscopy at Leica. “Scientists can now effectively scale their experiment down to the nanoscale using familiar protocols and a wide range of fluorescent labels and markers commonly used in biological research.”
Lessening the photodamage
STED is not without its drawbacks. Scientists have lamented the photodamage that has historically inhibited its use for live-cell imaging, which requires longer periods of observation. As the depletion beam intensifies, photobleaching occurs.
While this has shown promise in resolving nitrogen-vacancy centers — structural defects in diamonds that can be exploited for superresolution purposes — scientists have had to get creative in expanding its utility for time-lapse imaging, according to Kyu Young Han, an associate professor in the College of Optics and Photonics at the University of Central Florida. And he and his colleagues have been exploring how deep learning may help to overcome this technical hurdle.
“Even when you’re working with a fixed sample, photobleaching can be a big issue for STED, because you’re only capturing a limited number of photons at a time,” Han said. “So we studied what we could do in STED beyond the instrumentation.”
He said there are typically two ways deep learning can be used: cross-modality training of low-resolution from high-resolution images, and a de-noising method of low signal-to-noise ratio (SNR) from high SNR images. When exposure time is limited, it becomes essential to maximize the data gathered from these images.
In their study, they collected low SNR STED images at a short pixel dwell time as well as high SNR STED images at a longer pixel dwell time, acquiring images of microtubules in mammalian cells (Figure 3). For STED images, they used a Leica SP8 3X STED with an oil objective, or an Abberior STED Expert Line, and a 775-nm depletion beam. Following the testing, they determined that 20 image data sets provided ample information for training3.
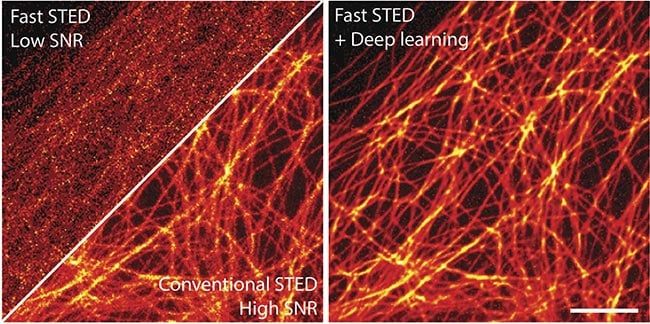
Figure 3. Deep learning enables fast and gentle stimulation emission depletion (STED)
imaging. STED images of microtubules in a U2OS cell. Scale bar: 2 µm. Courtesy of
Kyu Young Han/University of Central Florida.
Han explained that they used a two-step architecture, wherein a convolutional neural network collected images that were noisy; the second was a residual channel attention network dedicated to recreating the superresolution structural context.
“What we were looking at was that, even though the raw data has noise, it still has the high-resolution information that can be extracted,” he said.
Han said that they learned how powerful this approach could be during a collaboration with the Max Planck Institute, in which they monitored the dynamics of mitochondria, organelles within cells that provide the energy for multiple biological processes. Using equipment from both Leica and Abberior, they observed how these components changed structurally without photodamage and photobleaching.
“There really isn’t a huge library of STED images, so we want to show as much data as we can,” Han said. “STED has always been useful for fundamental research, but if we can classify the data quickly, even in 4D, I believe it could ultimately be applied in a clinical setting.”
References
1. S.W. Hell and J. Wichmann. (1994). Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy. Opt Lett, Vol. 19, No. 11, pp. 780-782.
2. M. Weber et al. (2023). MINSTED nanoscopy enters the Ångström localization range. Nat Biotechnol, Vol. 41, No. 4, pp. 569-576.
3. V. Ebrahimi et al. (2023). Deep learning enables fast, gentle STED microscopy. Commun Biol, Vol. 6, No. 1, p. 674.
Branching out from STED
As superresolution imaging methods have evolved over the years, companies that have provided the instrumentation for these techniques have had a bird’s-eye view of some groundbreaking research enabled by these systems. And this insight has allowed companies, such as Abberior Instruments, to move into increasingly specialized areas, revealing not only information about cellular structure, but also how different cells communicate with each other.
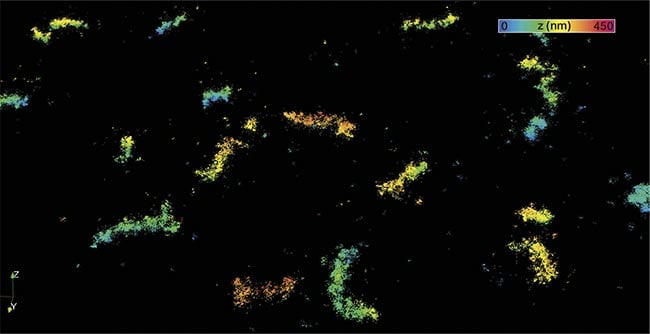
A MINFLUX image of peroxisome, membrane-enclosed organelles that contain enzymes for a variety of metabolic reactions. Courtesy of Abberior Instruments.
The research group headed by Nobel Prize winner Stefan Hell, who developed stimulated emission depletion (STED) microscopy, and Abberior have demonstrated that a technique called MINFLUX can be used to examine the structure of membrane proteins that regulate the delivery of genetic material between cells. They have been able to home in on the “blades” of PIEZO1 channels, which are mechanically sensitive ion channels that open in response to mechanical stimuli. They ultimately translate these mechanical signals into a process that leads to tissue inflammation.
MINFLUX, which is named after the concept of minimal emission fluxes, is a single-molecule localization microscopy technique, which shares some similarities with photoactivated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM), but with far greater localization precision. It uses a doughnut-shaped probing beam to triangulate fluorescent emitters, one at a time. The technique has been shown to differentiate fluorescent dye molecules ~5 nm apart, and has proved to be useful to study protein organization in both 2D and 3D imaging1.
Matthias Reuss, head of research and development and marketing at Abberior, stressed that while MINFLUX is related to other techniques such as PALM, STORM, and STED, it is a new technique. In contrast to all other superresolution methods, MINFLUX establishes the position of a molecule by looking for minimal emission instead of maximizing it. To this end, it uses the same doughnut hole beam as STED, but for the excitation of fluorophores. Then, the beam is iteratively moved until emission is minimal, at which point it exactly and measurably coincides with the position of the molecule. This allows fluorescent molecules to be localized and tracked while using only a very small number of precious fluorescence photons (see above image).
“Other techniques like electron microscopy can allow for sub-nanometer resolution,” Reuss said. “But with MINFLUX, you can follow fast-moving molecules with high precision. The beauty of MINFLUX is that it’s adaptive, meaning that you can get a first estimate of where the localization is happening with just a few photons, and adjust the illumination accordingly, making the following photons more informative, then adjust again, and so on.”
He said that the microscopes Abberior produces that can perform MINFLUX can also perform STED, but the former is where the company is focusing the most on building the market.
“Right now, there’s a huge interest in protein structures and dynamics in a number of disciplines, and MINFLUX is allowing that observation at outstanding spatiotemporal resolution,” Reuss said. “And we want more than just specialists to be able to do it.”
Reference
1. F. Köpper (August 2023). MINFLUX unravels the structural dynamics of PIEZO1 ion channels in living cells. Abberior Instruments GmbH, white paper, pp. 1-3.