Versatile light sources can be integrated into user-friendly automated instrumentation for high-throughput screening in cellular analysis.
Isabel Goodhand, CoolLED
Automation is a widespread trend across the life sciences, maximizing throughput and efficiency in applications such as drug development and clinical testing. For example, high-throughput screening — often enhanced by advanced illumination — accelerates the development of new drugs, while multiplex fluorescence imaging systems are helping to advance spatial biology research and the study of molecular interactions.
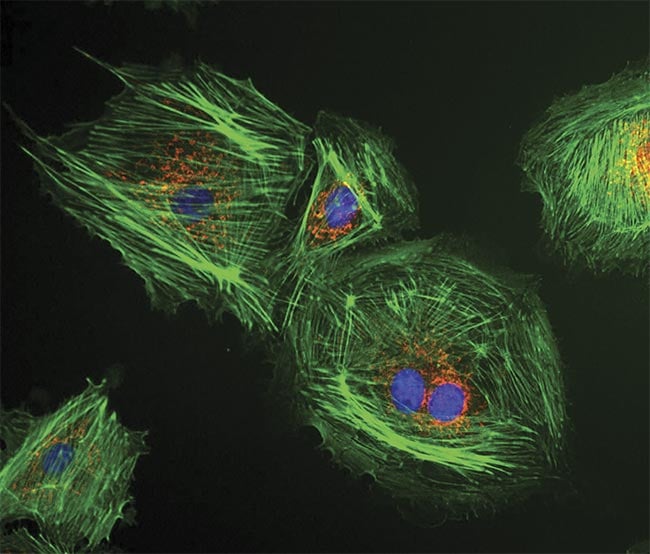
LED illumination excites the fluorescence from bovine pulmonary epithelial cells. Courtesy of CoolLED.
The availability of advanced technology, including sophisticated data analysis tools, combined with growing demand for high-efficiency laboratory solutions has intensified competition in the automated fluorescence microscopy market.
However, embarking on designing and manufacturing a new automated imaging system for fluorescence microscopy is a challenge that can quickly become an expensive and complex project. It is simply not feasible to manufacture many of the components that are integral to the microscope in-house. In contrast to the functionality of these other elements, the light source can seem relatively simple: produce a focused beam of light at a specified wavelength. Although it might seem entirely possible to design and manufacture this component, many aspects must be considered for a high-performance, customized fluorescence light source.
No two imaging systems have the same requirements, and the following issues must be addressed to augment the performance of the light source, and therefore the complete instrument.
Which light source?
While some systems rely on lasers, wide-field fluorescence microscopy is fast and affordable, which is ideal for automated setups. LEDs have become the technology of choice for wide-field fluorescence, rapidly growing in popularity as manufacturers work to make their instruments as adaptable as possible.
Compared with mercury and metal halide lamps traditionally used for wide-field fluorescence, LEDs offer many advantages for modern automated systems. Their long lifetimes maximize instrument uptime — gone are the days of replacing and realigning bulbs. A stable output over time also provides comparable data between experiments performed months or years apart, which is particularly valuable when monitoring long-term changes in cellular or tissue structures, such as neural degeneration in disease models. And due to their solid-state nature, the speed of electronic control translates to increased sample throughput. These are only a few key advantages, in addition to aspects such as superior energy efficiency and sustainability, user-friendly operation (simple control features), and low-cost maintenance, as opposed to expensive laser illumination or mercury lamps.
Spectral characteristics
Compared with mercury and metal halide lamps traditionally used for wide-field fluorescence, LEDs offer many advantages for modern automated systems.
Once LED technology has been chosen for a project, the next decision to make is which LED, or LEDs, to use. In order to excite the fluorophore set required by the end user’s application, many LEDs are available across the standard spectrum that is used in life sciences microscopy, from DAPI, a DNA-binding dye, with peak excitation at 365 nm; to Cy7, a red-shifted fluorophore for tagging many different biomolecules, with peak excitation of 740 nm (Figure 1).
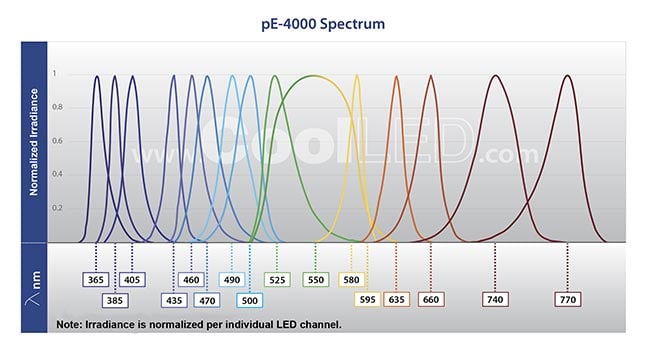
Figure 1. A selection of LEDs can be chosen to suit fluorophore requirements. This LED light source includes eight individual LEDs, which can be controlled as individual channels, or as one global channel. Courtesy of CoolLED.
For more specialized requirements, this range can extend down to 340 nm for Fura-2 calcium imaging — popular in measuring calcium dynamics in neuronal activity — or up to 850 nm in the near-infrared. Red-shifted fluorophores are becoming more popular, because the lower energy at this end of the spectrum reduces the risk of phototoxicity and photobleaching, improving data accuracy during live-cell analysis.
While a light source can house many individual LEDs, how these are controlled can vary. The simplest option is to control all LEDs globally, on one channel, minimizing costs for single-color imaging applications, such as cell viability studies to screen for cytotoxicity properties of a compound. Alternatively, each LED can be controlled individually, with such multichannel LED light sources better suited to multicolor imaging. Multicolor imaging has many diverse applications but is especially valuable in spatial biology applications, for example, when characterizing the tumor micro-environment or mapping neuronal circuits. The ability to switch between channels increases the speed of acquiring high-quality images when used with a multiband or Pinkel optical filter configuration (Figure 2).
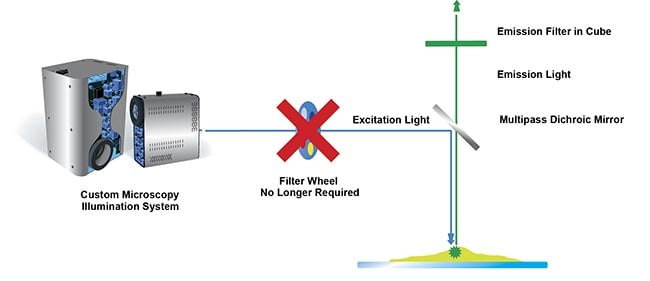
Figure 2. Faster imaging with individual channel control. Using single-band excitation filters housed within the light source and a multipass dichroic and emission filter, individual LED channel switching achieves fast and high-quality imaging.
Courtesy of CoolLED.
Not all LEDs have the same spectral width, and even if the peak of an LED does not exactly match the peak absorption of a fluorophore, it may still be compatible.
To maximize image quality (including spectral separation in the case of multicolor imaging), the user must pay close attention to exactly which fluorophores will be required, their absorption spectra compared to the LED spectra, and compatible filter sets. Not all LEDs have the same spectral width, and even if the peak of an LED does not exactly match the peak absorption of a fluorophore, it may still be compatible. This is the case when using a popular blue LED wavelength centered at 450 nm to excite enhanced green fluorescent protein (EGFP), a fluorophore that has an absorbance peak of 488 nm (Figure 3).
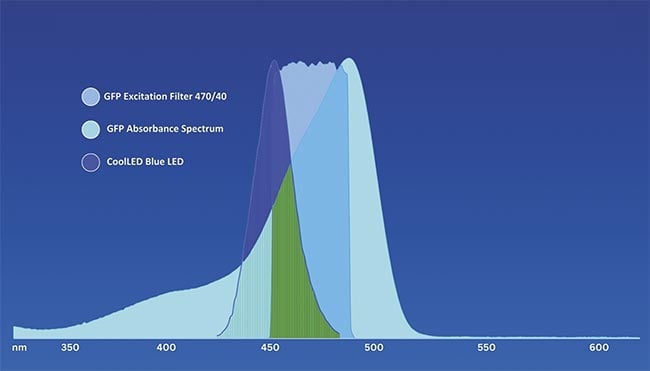
Figure 3. Enhanced green fluorescent protein (EGFP) compatibility with CoolLED LED illumination systems: Matching fluorophore absorbance, excitation filter specification, and an LED spectrum. In this example, the LED center wavelength and GFP peak absorbance do not exactly match. However, due to the broadness of the LED emission, there is sufficient overlap for GFP excitation (green). Courtesy of CoolLED.
Control and connectivity
LED illumination is often sought after for the range of electronic control options possible, which enhances the performance, flexibility, and reliability of automated imaging systems.
For example, the light source can be synchronized with other components in the light path, such as the camera, and these settings can be saved and repeated for precise and reproducible automated imaging with minimal operator input.
The challenge of developing an optimized LED light source underscores the importance of determining detailed requirements early in the project.
Electronic control is also fast compared to traditional mechanical shutters. Each channel (or all channels simultaneously in the case of global control) can be switched on or off with near-instant speed. Fast imaging does not just increase sample throughput; it can capture dynamic samples in greater detail, in the case of live-cell imaging applications. Förster resonance energy transfer experiments especially benefit from high-speed imaging, capturing transient signals with high temporal resolution, and providing more data points to resolve fast biomolecular interactions; this enables accurate quantification of dynamic molecular processes, such as signaling pathways.
To fine-tune brightness, either between samples, or even individual fluorophores within the same image, percentage irradiance modulation is possible individually or globally, although the electronic engineering involved can be highly complex to ensure linearity.
Electronic control options include USB for software control. In addition, RS-232 and Ethernet are returning to popularity as manufacturers value their robust nature in automated applications, such as high-throughput antibody screening, in which a combination of speed and stability is crucial. Transistor-to-transistor logic is the technology of choice for ultrafast LED triggering, while analog also allows for ultrafast irradiance control.
Light delivery
A few different options are possible for interfacing the light source to the imaging system, while optimizing both the mechanical coupling and optical configuration. To enhance irradiance at the sample plane, direct coupling is sometimes possible depending on the spatial constraints within the instrument, and the optics must be carefully designed to ensure optical homogeneity. A liquid light guide connection provides flexibility in terms of placement within or outside the instrument housing, but the drawbacks are twofold: Reduced irradiance occurs at the sample plane compared with direct coupling, and UV damage to the liquid light guide over time will require its replacement. Optical fibers, on the other hand, are ideal for focusing the beam of light and maximizing irradiance in a pinpoint area, but they also reduce irradiance.
Optical design
The light hitting the sample plane must have sufficiently high irradiance to excite the required fluorophores, and the homogeneity to ensure precise sample analysis across the field of view. Homogeneity is especially important for image-stitching applications (used for detailed analysis of large tissue samples), to avoid vignetting and to ensure a smooth transition between individual tiles.
There are trade-offs to be made between irradiance and homogeneity, and the property of etendue (the ability of a source to emit light and the ability of an optical system to accept light) is crucial to understanding this. Defining the angle and spread of light emitted from the LED, etendue therefore defines how much power can be collected by the optical system, focused efficiently and transmitted to the sample plane. The wider the angle, the higher the homogeneity, but this comes with a loss of irradiance — and vice versa.
Another consideration of optical design is that the coating of optical filters is angle-dependent and compatible with light beams of up to ~10°. Collimation of the light source is vital for correct optical filter compatibility.
Size and thermal constraints
Automated imaging systems often work their components hard, especially in the clinical pathology setting for pathogen detection, or human epidermal growth factor receptor 2 (HER2) screening in the case of cancer investigations. Samples can be running around-the-clock with many millions of images acquired per year.
Driving LEDs hard generates more power, but it also generates heat (Figure 4). Unless properly managed, this can severely limit the LED’s stability and lifetime. Thermal management is therefore a key part of any LED light source design and a highly demanding area of expertise. A combination of large heatsinks, space, and fan cooling systems are essential.

Figure 4. The importance of thermal management in LED illumination. As an LED (central square) is driven to maximize power, it also generates heat — in this case, up to 94.1 ºC — which must be managed to ensure LED stability and a long lifetime. Courtesy of CoolLED.
Of course, these thermal management solutions add to the size of the light source, which is a consideration when fitting the light source inside an automated imaging instrument. Size constraints of the light source can often be an afterthought for system builders, only considered toward the end of a project. But the thermal control components in addition to the number of LEDs (and therefore optical path lengths) all affect the final size of the light source, and should be considered early in the design phase.
Purchasing LEDs
Catalogs offer countless LEDs to choose from, which are becoming increasingly powerful. One additional aspect to consider is that each production round of LED manufacture results in performance deviations, including in wavelength and light output. Purchasing low quantities of LED chips directly from a component reseller will inevitably produce variance, that is inherent in LEDs from different bins; for example, wavelength variance of up to 25 nm is not uncommon. It is possible to reduce this spread to <5 nm, and specialist manufacturers of LED light sources achieve this by purchasing in bulk directly from the manufacturer at bare LED die level, and then performing internal testing.
As the life sciences, and even applications outside the life sciences, such as semiconductor inspection, enjoy the advantages of automated fluorescence systems, instrument manufacturers are keeping pace with the latest technology trends. The considerations that are important for the developer and the user include the light source, which can sometimes be overlooked. However, fluorescence microscopy illumination is far from simple, considering the complexities inherent in the sample and the details that the user aims to extract. The challenge of developing an optimized LED light source underscores the importance of determining detailed requirements early in the project.
Treating the light source as an afterthought can lead to costly redesigns and compromised performance. With numerous considerations to address — from spectral compatibility and optical design to thermal management and control — careful planning from the outset is essential to navigating the challenges and delivering a robust, high-performance system in an increasingly competitive market.
Meet the author
Isabel Goodhand, Ph.D., technical content specialist at CoolLED, completed her doctorate in 2011 at the University of Portsmouth in England, where she developed a method to quantify protein-DNA binding activity. Since leaving academia, she has specialized in scientific communications across the science and engineering sectors. She joined CoolLED in 2019 and helps to keep the microscopy community up-to-date with the latest developments in LED microscopy illumination; email: [email protected].