Photonics HandbookFeatures
Feeling with Photons: Brillouin Microscopy Advances Biomaterials Research
By examining the mechanics and viscosity of cells, Brillouin microscopy helps elucidate the fundamental processes in disease progression.
By Torsten Jähnke
Brillouin microscopy was first developed for materials science fields that focus on the examination of condensed matter to assess properties such as elasticity and viscosity. The introduction of Brillouin microscopy to the biological sciences has improved the analysis of biomechanics, because it can directly image the viscoelastic properties of living biological matter. While many imaging techniques have preceded Brillouin microscopy, they cannot effectively probe viscoelastic properties of cellular matter, because they either require establishing a direct contact with the sample, therefore damaging it, or lack the required 3D resolution to properly comprehend the material’s characteristics.
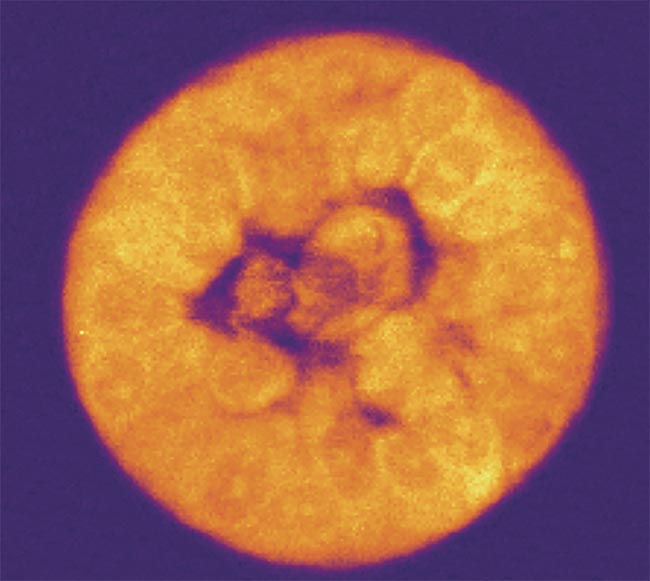
An example of a Brillouin shift, defined as a frequency shift of light that illuminates the elasticity and viscosity of a sample. Courtesy of CellSense Technologies.
Around 20 years ago, Brillouin microscopy emerged as a form of optical elastography that was developed from non-scanning high-resolution optical spectrometers and is currently seeing a rapid expansion for the characterization of soft matter. Brillouin microscopy is a nondestructive, label-free, and contact-free 3D-imaging method that can identify a biomaterial’s mechanical properties at the cellular and subcellular level. These properties are important for diagnosis, because they can lead to understanding how the local biomechanics of cells can contribute to the progression of diseases.
Brillouin microscopy is currently used to investigate the intracellular biomechanics of living cells, analyze the phase transitions in individual cellular structures, and assess the biomechanics of tissues. This information can serve as an early warning tool for the detection of different diseases — such as keratoconus, atherosclerosis, amyotrophic lateral sclerosis, meningitis, and Alzheimer’s disease. More recently, Brillouin microscopy has established itself as an effective tool for cancer research.
The basics of Brillouin
Brillouin microscopy’s potential can be found in its ability to investigate a material’s viscosity, elasticity, and micromechanical properties by measuring its longitudinal modulus in the gigahertz frequency range. The technique relies on the principle of Brillouin light scattering and is similar in nature to other optical imaging methods, such as confocal microscopy.
Brillouin light scattering is an inelastic process that occurs when light interacts with acoustic waves in a material. These acoustic waves originate from the collective vibrations of atoms and molecules in solids and liquids. In solid-state physics, these vibrations are well known as phonons, which describe lattice vibrations that reflect a material’s mechanical and thermal properties.
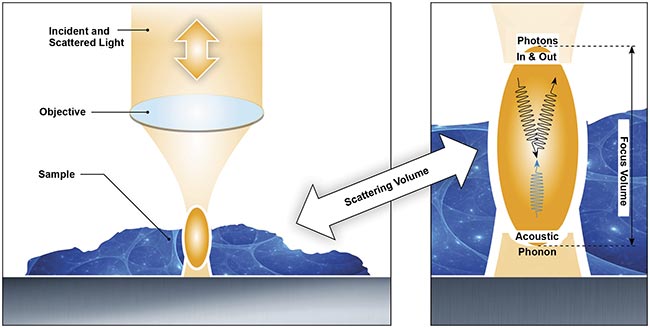
The process of Brillouin light scattering, the mechanism that makes Brillouin microscopy an effective tool for investigating viscoelasticity
with optical resolution. Courtesy of CellSense Technologies.
In spontaneous Brillouin scattering, light is scattered due to natural thermal fluctuations in the material, providing insights into its viscoelastic properties. Unlike stimulated Brillouin scattering, which requires intense laser fields to amplify the interaction, spontaneous Brillouin scattering enables noninvasive, label-free mechanical characterization of biological and synthetic materials.
In Brillouin microscopy, an illumination source — normally a narrowband frequency laser — sends a beam of photons directly to the sample light that is scattered elastically from a sample, called Rayleigh light, which has the same frequency and wavelength as the illumination source. However, a small fraction of the incident laser light is inelastically scattered from phonons propagating in the longitudinal (axial) directions.

The Discoverer Brillouin microscope from CellSense that is used to noninvasively measure the viscoelasticity of live samples. Courtesy of CellSense Technologies.
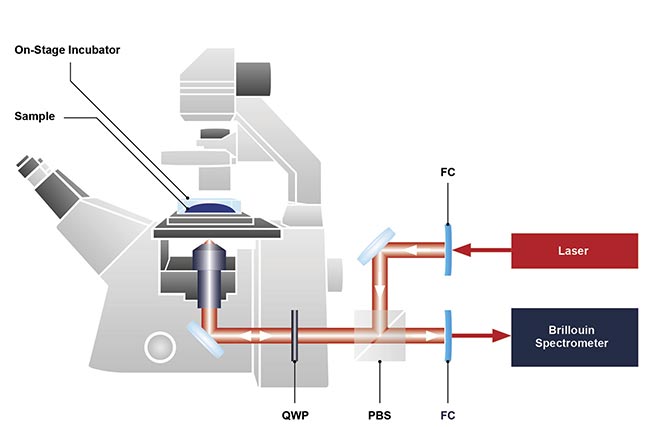
The basic design of a Brillouin microscope integrated with a conventional inverted optical microscope. FC: fiber coupling; PBS: polarizing beam splitter; QWP: quarter wave plate. Courtesy of CellSense Technologies.
The phonons behave like a diffraction grating, making the waves undergo a frequency shift, known as a Brillouin frequency shift (BFS), similar to the Doppler effect, in which the frequency of waves changes in relation to the speed and direction of their interaction. The frequency of the scattered light shifts by the exact value of the acoustic frequency. This frequency shift creates two peaks in the scattered light spectrum known as Stokes and anti-Stokes Brillouin peaks. The energy transfer from the photon to the phonon results in a downshifted Stokes peak, whereas the energy transfer from a phonon to a photon generates an upshifted anti-Stokes peak.
Brillouin insights in biology
The shifted Brillouin peaks have specific values depending on the tissue being imaged (such as neural or muscle tissue), the wavelength of laser light used to image the sample, and the scattering geometry. If the material has a known density and refractive index, the viscoelastic properties of a material can be identified because the BFS is linked to the elastic response of the material. The velocity and attenuation of the soundwave have an intrinsic dependence on the viscoelastic properties of the material.
The analysis of the BFS is performed by high-resolution (sub-gigahertz) spectrometers with highly sensitive detectors or cameras. These systems can also be coupled with Raman spectrometers and fluorescence microscopes to look at the morphology, structure, and biochemical properties of the sample in more depth. In recent years, Brillouin microscopy has been combined with traction force microscopy to look more deeply at cellular biomechanics and cell-generated forces.
Scientists use Brillouin microscopy to build 2D and 3D images by acquiring BFS spectra at different points within the sample. Each of these points represents a pixel of the image, and these pixels contain information on the local stiffness and viscosity of the sample at that imaging point. Users of the microscope obtain a data cube filled with mechanical property details in 3D once all the mapping has been performed.
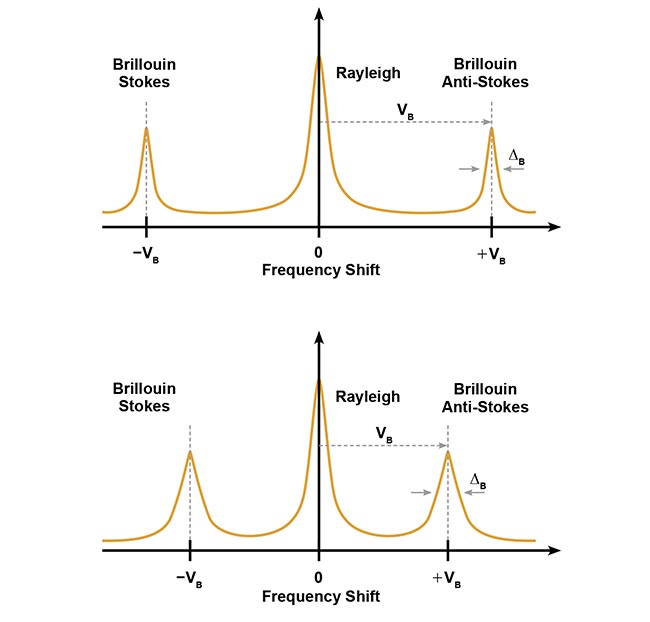
A graphical illustration of Stokes and anti-Stokes shifts, reflecting the nature of energy transfer in materials with different bulk elastic modulus. Courtesy of CellSense Technologies.
These 3D images generated by Brillouin microscopy enable the local micromechanical properties of the biomaterial to be characterized, leading to the identification of different structures in the sample, such as the extracellular matrix or the nuclei as well as morphological characterization, and the visualization of cells, cell aggregates, and even subcellular compartments.
Importance of viscoelasticity
At the single-cell level, mechanical forces and changes in viscoelastic properties within both extracellular and intracellular environments play a key role in how cells and tissues function. Significant progress has been made during the last three decades in mechanosensing and mechanotransduction mechanisms, i.e., the cellular mechanisms that sense and respond to mechanical stimuli. This has led to a greater understanding of the major role that mechanical forces play in the development of many pathologies, ranging from cancer to cardiovascular disease and how viscoelasticity is an indicator of disease progression.
Scientists and clinicians have come to understand that the mechanical properties of all cellular matter and tissues are responsible for different biological functions inside the human body. At the cellular level, the elastic and viscosity properties modulate the differentiation and migration of cells as well as determine how the cell will respond to different physical forces exerted in its local environment. For example, changes in cell stiffness have been linked to an increased aggressiveness in tumors, whereas the elasticity of the extracellular environment plays a role in directing stem cells along specific lineages when implanted in the body. The elasticity of the extracellular environment can also dictate the progression of tumors.
At a more technical level, the longitudinal modulus — which is different from Young’s modulus (the linear part of the stress-strain curve) and only involves a 1D stress/strain state — provides information about the elastic properties of biological components. Information that is obtained by probing the viscoelastic properties of biomaterials includes the compressibility of the material and the solid-liquid volume fraction. This is important for understanding cellular environments, where the viscoelastic response of the cell is governed by both the solid components and the liquid fraction.
While mechanical forces influence biological behavior at the cellular level, the mechanical properties of tissues are also important for understanding the progression of different diseases. It is understood that a tissue’s mechanical properties are one of the key factors for determining how cells organize to form the tissue itself and the morphogenesis of the tissue — the process that shapes the tissue to meet its intended function. The extracellular environment can be seen as elastic networks surrounded by fluid. Extracellular environments can facilitate the adhesion of cells to each other, creating a network of solid meshworks. Obtaining regulation of the cross-linking, branching, and solid-liquid fraction properties of the network in the extracellular environment is key for tissue growth. Therefore, understanding these mechanisms from a mechanical and viscoelastic perspective is crucial for understanding the formation and progression of various morphological diseases, such as cancers, atherosclerosis, and eye diseases.
Application areas
In the field of cell mechanobiology, mechanotransduction interactions between cells and their microenvironment are important for sensing mechanical stimuli in the extracellular environment and converting them into biochemical signaling. Mapping the intracellular and extracellular elastic modulus distribution has helped scientists to better understand how forces are transmitted in a cell. Brillouin microscopy meets the necessary resolution requirements for mapping the subcellular scales in 2D and 3D to better understand the cell mechanics in play. It is used to investigate actin polymerization, branching and remodeling, and cell adhesion properties as well as extra- and intracellular stiffness changes.
Brillouin microscopy could also potentially be employed to assist in the diagnosis of a range of diseases, including ocular, cardiovascular, bone, dentin, and cartilage disease. In ocular applications, Brillouin microscopy is helping scientists to characterize the corneal mechanics for keratoconus. Keratoconus causes the corneas to have a lower mechanical strength due to fewer cross-links than mechanically strong healthy corneas, and Brillouin microscopy can be used to distinguish between the two.
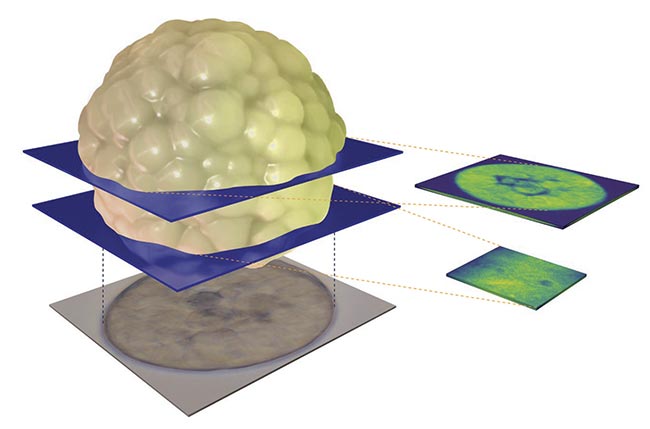
Brillouin microscopy can ultimately provide 3D images of samples as well as their material properties. Courtesy of CellSense Technologies.
In cardiovascular research, Brillouin microscopy can analyze the viscosity and elasticity of heart tissue to identify any stiffness changes that correspond to cardiovascular disease. The heart muscle shortens and generates forces that are disturbed if the heart is diseased. For bone, dentin, and cartilage, their mechanical properties are vital to their function, but the disruption of these properties, which can be detected with Brillouin microscopy, is a key marker for various bone diseases, including osteoporosis and arthritis.
Cancer research
Cancer cells often exhibit altered biomechanical properties, when compared with healthy cells. These mechanical changes can provide insight into tumor aggressiveness, as studies have shown that increased cell deformability may correlate with a higher metastatic potential (the ability of cancer to spread from its primary location).
Traditionally, the mechanical properties of cancer cells have been studied using techniques that require isolating single cells from cultures or tissues. However, these methods do not capture how cancer cells behave in their natural 3D environment. In contrast, Brillouin microscopy provides a noninvasive approach to studying cancer cell mechanics in more relevant physiological conditions, such as 3D tumor models or small tissue fragments.
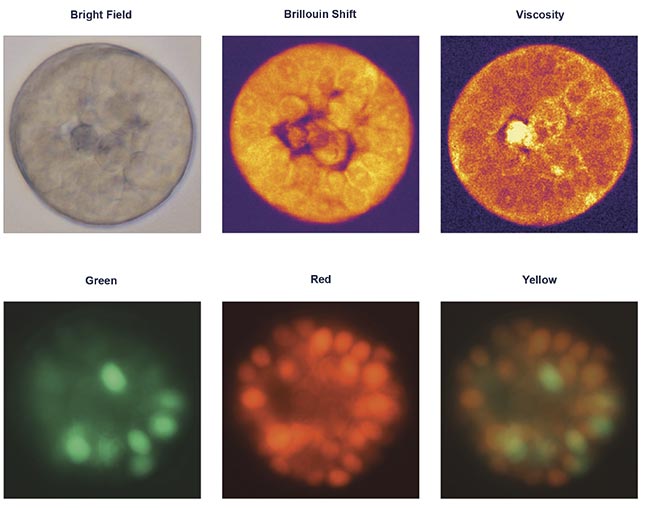
Brillouin measurements can provide clear illustrations of elasticity and viscosity, which are otherwise inaccessible, in addition to and in correlation with other optical contrast methods. Courtesy of CellSense Technologies.
Recent research from the Taubenberger group at Dresden University of Technology has demonstrated the potential of Brillouin microscopy for characterizing the mechanical properties of 3D in vitro tumor models. In this study, the researchers analyzed MCF-7 breast cancer cells forming tumor spheroids within an extracellular matrix-mimicking hydrogel. MCF-7 cells are widely used in breast cancer research because they closely resemble the behavior of estrogen-responsive tumors.
For this study, MCF-7 cells were cultured in a hydrogel for 12 days. Because the cells expressed a cell cycle reporter, called fluorescence ubiquitin cell cycle indicator (FUCCI), their progression through the cell cycle could be tracked in real time. The researchers observed that in compliant hydrogels (1 to 2 kPa), certain spheroids developed a central lumen — a structural feature of mammary morphogenesis that is typically lost in tumorigenesis. Imaging at multiple depths, both through the center and along the outer edge, provided a detailed 3D representation of a live organoid.
Because Brillouin microscopy works on live samples, the researchers could monitor structural changes that occurred over time. The FUCCI reporter enabled them to track cell cycle progression while simultaneously measuring how biophysical properties evolved at different stages. Additionally, the researchers observed how cells proliferate and organize in response to mechanical and biochemical signals from their microenvironment.
A deeper understanding of these 3D tumor models, particularly their mechanical properties and structural organization, is considered by many researchers to be crucial for developing new targeted therapies. By providing noninvasive, real-time insights into tumor biomechanics, Brillouin microscopy could help advance personalized medicine and the development of precision cancer treatments.
Many characterization techniques that possess an adequate resolution must physically touch a biomaterial to analyze its mechanical and viscoelastic properties, damaging and contaminating it in the process. In contrast, Brillouin microscopy has emerged as an effective noncontact, nondestructive, and high-resolution technique in the life sciences.
Meet the author
Torsten Jähnke is chief technology officer at CellSense Technologies GmbH. He was one of the founders of JPK Instruments, a pioneering company in the microscopy field, and successfully steered it to a strategic acquisition by Bruker in 2018. In his current role at CellSense, Jähnke’s visionary leadership continues to shape the trajectory of Brillouin microscopes, contributing to groundbreaking developments in mechanobiology instrumentation; email: tj@cellsense-technologies.com.
/Buyers-Guide/CellSense-Technologies-GmbH/c34034