By spectroscopically measuring the temporal fluctuations of scattered light from the eye, clinicians can determine the presence of amyloid in the brain without a traditional scan.
Jeffrey Weiss, Micron Ophthalmic
Alzheimer’s is a slowly progressing disease caused by physical changes in the brain, resulting in the loss of mental abilities and memory. Unfortunately, a simple clinical test does not exist to diagnose the condition in a living patient. Currently, clinical history, mental status examination, and a PET scan to determine the presence of amyloid is used to diagnose the condition. The definitive diagnosis is made posthumously during an autopsy, through the discovery of protein fragment beta-amyloid plaques and twisted strands (tangles) of the protein tau. But optical phenomena such as dynamic light scattering (DLS) reflective of subtle cellular changes could potentially alter this diagnostic timeline dramatically.
An illustration of the human brain with Alzheimer’s disease. Courtesy of iStock.com/Lars Neumann.
The average life expectancy following the presumed diagnosis is five to 10 years. A successful treatment has not been identified to date, although in some cases, pharmaceuticals may slow progression and alleviate symptoms.
During the last 15 years, more than 500 clinical trials of therapeutic agents for Alzheimer’s disease have been registered with the National Institutes of Health website ClinicalTrials.gov. For trials with reported results, the failure rate has been almost 100% in slowing or stopping the disease’s progression. Though most clinical trials have typically lasted one and a half to three years, a study duration of five years may have been required to detect a definitive clinical effect, by medical standards.
The eye is the only place in the body where an artery, vein, and nerve can be directly visualized. The nerve fiber layer of the retina is an extension of the brain. It has been demonstrated by ocular coherence tomography (OCT) imaging that patients with Alzheimer’s disease have thinning of the retinal nerve fiber layer and retinal ganglion cell layer, consistent with histopathologic data. Inner retina thinning has been correlated with disease severity. It is apparent that early changes at the molecular level must lead to much later changes visible in imaging.
The basics of DLS
DLS spectroscopy — also known as photon correlation spectroscopy, quasi-elastic light-scattering spectroscopy, or laser light-scattering spectroscopy — measures the random thermal movement (a phenomenon known as Brownian motion) of particles in a solution by analyzing the temporal fluctuations in scattered light intensity. The random motion of proteins causes localized changes in particle concentration, which affects the intensity of scattered light.
The scattered light intensity I(t) is compared to the scattered light intensity at a later time, which is measured as a time correlation function using the following formula:
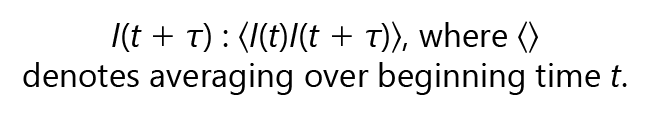 Scattered wave interference generates a net scattered light intensity I(t), which displays stochastic fluctuations, depending on whether the interference is constructive or destructive, due to the random motion undergone by suspended particles. The DLS correlation curve assumes that each detected photon has been scattered once.
Scattered wave interference generates a net scattered light intensity I(t), which displays stochastic fluctuations, depending on whether the interference is constructive or destructive, due to the random motion undergone by suspended particles. The DLS correlation curve assumes that each detected photon has been scattered once.
There is a high correlation between the signal and short time delays, as the signals are unchanged because the particles do not have a chance to move significantly between the light source and the detector. With a longer time delay, the correlation will decay exponentially. A monodisperse sample (one with particles of a uniform size) will exhibit a single exponential decay. This pattern is captured in the following equation:

The translational diffusion coefficient Dt may be derived at a single angle or at a range of angles, depending on the wave vector q:
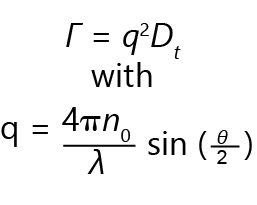 In this equation, λ is the incident laser wavelength, n0 is the sample’s refractive index, and θ is the angle at which the detector is located with respect to the sample.
In this equation, λ is the incident laser wavelength, n0 is the sample’s refractive index, and θ is the angle at which the detector is located with respect to the sample.
Small spherical particles do not demonstrate angular dependence or anisotropy. Nonspherical particles demonstrate angular dependence and anisotropy. An optimal angle of detection exists for each particle size. This is important in a polydisperse sample with an unknown particle size distribution. The autocorrelation function is a sum of the exponential decays corresponding to each species in the population. From this calculation, the state of decay of various cellular components can be determined.
Historically, DLS has been used to predict the development of cataracts in rabbits1, the development of cataract formation and diabetes mellitus in humans2-4, the effectiveness of treatment for wet age-related macular degeneration5, and the success of retinal stem cell surgery6.
The results have demonstrated the utility of DLS to noninvasively quantitate subtle changes at the molecular level. DLS captures molecular changes that are indicative of a particular disease or treatment earlier than they could be captured by imaging methods that detect late changes in structure.
A proof-of-concept instrument for making retinal measurements has been developed (Figure 1). The detector is interfaced with a standard clinical fundus camera. Scattered light is analyzed by a digital autocorrelator with an extended delay option for baseline determination. The intensity fluctuations are averaged over 5 s, and the cumulant analysis method is used to analyze the light-scattering data as a function of the sample time.
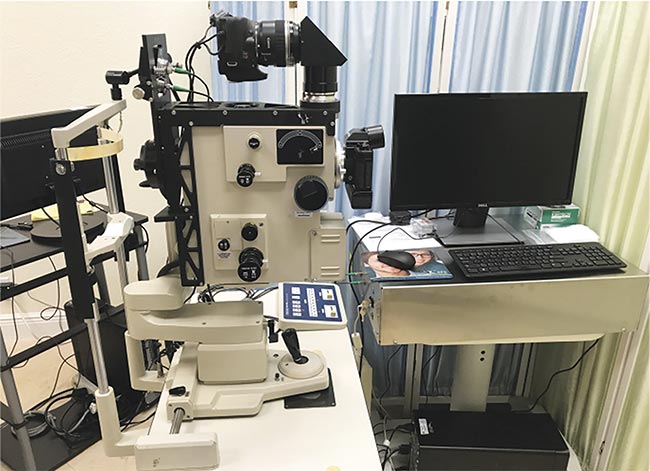
Figure 1. A prototype of the new clinical dynamic light-scattering (DLS) device. Courtesy of Jeffrey N. Weiss.
The instrument was used to test the eyes of 17 patients7,8 who had no history of neurologic disease. No significant differences were seen between the left and the right eyes in patients with normal eye examinations. In addition, the results, taken over a six-month time span, were not significantly different, demonstrating reproducibility and consistency of baseline measurements. The patients’ age had no significant effect on the measurement.
DLS measurements were then made from the macular area of 15 patients diagnosed with mild cognitive impairment and from a control group of 15 patients of similar ages with no history of neurological disease and with normal retinal examinations. Four patients were diagnosed with Alzheimer’s disease by DLS testing that were later diagnosed by standard clinical methods one to one and a half years later.
Could DLS predict the development of Alzheimer’s disease before the appearance of symptoms? If so, then the possibility exists for the development of drugs that can prevent the development of the disease with its attendant devastating symptoms.
Diagnostic potential
Next, DLS testing was performed on 16 patients with suspected Alzheimer’s disease undergoing beta-amyloid PET imaging, to determine amyloid deposition in the brain. To perform PET imaging, a radionuclide is injected, the patient waits ~90 min, and the test is performed. Many insurance companies do not cover the PET amyloid test, which costs approximately $5000. So DLS could be more cost-effective.
In the recent test of those with suspected Alzheimer’s disease, 10 of 16 (63%) patients tested positive for amyloid, and 6 of 16 (38%) tested negative. DLS testing correctly identified all 10 of 16 (100%) of the patients that were positive for amyloid, and 5 of 6 (83%) patients that were negative for amyloid (Figures 2-4). One patient exhibited a positive DLS measurement but a negative PET amyloid scan and will presumably be diagnosed with Alzheimer’s disease by standard clinical methods in the future.
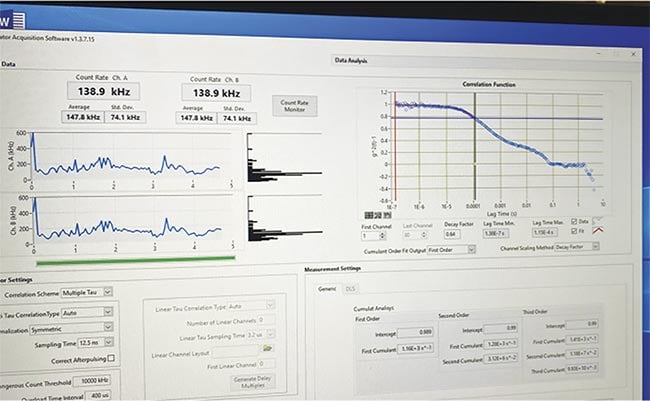
Figure 2. A screenshot of the dynamic light-scattering (DLS) test of a 74-year-old female with mild cognitive impairment and suspected Alzheimer’s disease. The PET scan was positive for amyloid protein deposition in the cerebral cortex. Courtesy of Jeffrey N. Weiss.

Figure 3. A screenshot of a dynamic light-scattering (DLS) measurement of an age-matched control patient with no Alzheimer’s symptoms. Courtesy of Jeffrey N. Weiss.
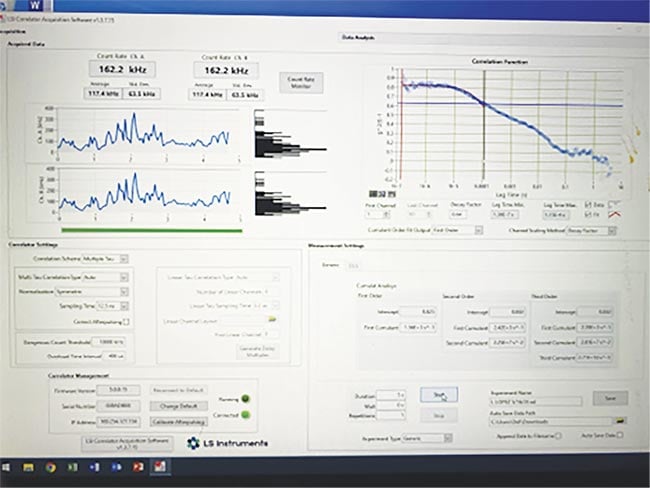
Figure 4. A dynamic light-scattering (DLS) test of a 73-year-old female with cognitive impairment. Positive PET for amyloid protein deposition in the cerebral cortex. Alzheimer’s disease measurements consistently begin above 0.6 g2(t)−1 and exhibit flattening of the initial line, representing significant changes in the structure of the eye, as opposed to the curve seen in patients without Alzheimer’s. Courtesy of Jeffrey N. Weiss.
The correlation between retinal protein changes, as determined by DLS ocular measurement, and beta-amyloid protein deposition in the brain could provide a sensitive test in early Alzheimer’s disease detection. DLS could provide an earlier diagnosis than PET amyloid scans or blood testing. DLS is inexpensive and noninvasive, and it provides immediate quantitative results. At present, three new instruments are being manufactured, and discussions are ongoing with multiple Alzheimer’s disease centers to perform large-scale clinical testing.
The development of an early, noninvasive, quantitative test to diagnose Alzheimer’s disease has the potential to lead to breakthroughs in drug development and, hopefully, to the successful treatment of patients before dementia is irreversible.
Meet the author
Jeffrey N. Weiss, M.D., is president of Micron Ophthalmic Inc. He is the former chief of retinal surgery at the Joslin Diabetes Center in Boston, faculty member of Harvard Medical School, and a visiting scientist at the Massachusetts Institute of Technology. Weiss is the author of Dynamic Light Scattering Spectroscopy of the Human Eye; email: [email protected].
References
1. I. Nishio et al. (1984). In vivo observation of lens protein diffusivity in normal and x-irradiated rabbit lenses. Exp Eye Res, Vol. 39, No. 1, pp. 61-68.
2. J.N. Weiss et al. (1984). Laser light scattering spectroscopy of in vivo human lenses. Invest Ophthalmol Vis Sci, Vol. 25, No. 5, pp. 594-598.
3. J.N. Weiss et al. (1986). Photon correlation spectroscopy of in vivo human cornea. Cornea, Vol. 5, No. 1, pp. 19-24.
4. S.E. Bursell et al. (1989). Clinical photon correlation spectroscopy evaluation of human diabetic lenses. Exp Eye Res, Vol. 49, No. 2, pp. 241-258.
5. J.N. Weiss (2013). Dynamic light scattering technique shows potential in measuring effects of AMD treatment. Ocular Surgery News, Retinal Ed., Vol. 31, No. 8, p. 57.
6. J.N. Weiss and S. Levy. (2019). Dynamic light scattering spectroscopy of the retina — a non-invasive quantitative technique to objectively document visual improvement following ocular stem cell treatment. Stem Cell Investig, Vol. 6, p. 8.
7. J.N. Weiss. (2023). Dynamic light-scattering method could guide Alzheimer’s diagnosis and treatment. BioPhotonics, Vol. 30, No. 2, pp. 46-49.
8. J.N. Weiss (2024). Alzheimer’s Disease and the Eye. Springer.